Geology Reference
In-Depth Information
π
electron density
Oxygen
Carbon
Ca
Crystallographic
symmetry axis (triad)
O
C
Light vibrating this way can polarise
π
orbital-high refractive index
(a)
Light vibrating this way cannot polarize
π
orbital
so experiences a lower refractive index.
(b)
Figure 8.5
Structure of calcite showing (inset) the orientation of sp
2
-based trigonal planar CO
3
2−
oxy-anion
. The highly polarizable
concentrations of
π
electron density perpendicular to the
z
-axis are shown stippled. Light vibrating in plane (a) encounters a high
refractive index. Light vibrating parallel to the
z
-axis (b) encounters less polarization and therefore a lower refractive index.
all three. Y-shaped
π
molecular orbitals therefore exist
above and below the plane containing the nuclei. To an
electric field oscillating perpendicular to the
z
-axis rep-
resented by the dashed arrow (Figure 8.5a), the anion
looks highly polarizable, because delocalized electron
density is easily shunted from one end of these
π
-
orbitals to the other by the oscillating electric field.
Light having this electric vibration direction therefore
experiences a relatively high refractive index (1.66).
The polarizability parallel to the
c
-axis is much lower,
so light vibrating in this direction encounters a lower
refractive index (1.49). Therefore when a mark on a
piece of paper is viewed through a clear cleavage
rhomb of calcite, two separate images are seen.
The majority of silicate minerals are also optically
anisotropic for similar reasons, although the degree of
anisotropy (the magnitude of the birefringence) is
much lower than for carbonates.
Crystal growth
The first step in the production of a crystal from a
surrounding liquid (melt or solution) is
nucleation
, the
formation of the initial embryonic speck of ordered crys-
talline material upon which the rest of the crystal will be
deposited. The free energy of this
nucleus
consists of:
(a) a negative term proportional to its volume, reflect-
ing the cohesive forces between close-packed ions/
atoms in the interior; and
(b) a positive term proportional to the surface area,
reflecting the reactivity of unsatisfied bonding
potential on the surface.
Thus for a cubic nucleus of edge-length
r
:
3
6 σ
∆
Gr Lr
L
=−
+
v
s
where Δ
G
L
is the free energy of the nucleus relative to
an equivalent amount of melt.
L
v
is the free energy of
fusion per unit volume, and
σ
s
is the surface energy per
unit surface area (Figure 8.6).
The initial nucleus, having a high surface area:volume
ratio, is therefore highly unstable, and it must grow
rapidly into a larger, more stable crystal if it is not to be
Defects in crystals
The extreme order of the crystalline state conceals the
fact that all real crystals incorporate structural defects,
which have a profound effect on the growth of crystals
and on their mechanical strength.










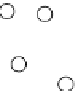

































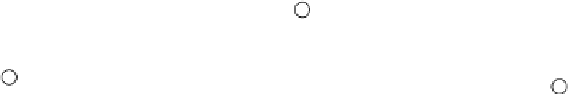















































Search WWH ::

Custom Search