Geology Reference
In-Depth Information
new structure of different symmetry. Because Mg
2
Si
2
O
6
and CaMgSi
2
O
6
have intrinsically different structural
arrangements, there cannot be a continuous series of
compositions between them. There is a
miscibility gap
between these minerals except at high temperature, with
the consequence that two pyroxenes of different comp-
osition can exist in the same rock in equilibrium with
each other. A similar gap operates in the alkali feldspars
between albite (NaAlSi
3
O
8
) and orthoclase (KAlSi
3
O
8
).
The compositions of co-existing feldspars (or pyroxenes)
lie on a
solvus
curve, as illustrated in Figure 2.6.
relevant to optical phenomena in crystals, because the
progress of light in a crystal depends upon the inter-
action between the electric disturbance and atomic
electron clouds. The extent of interaction, which is
measured by the refractive index of the crystal,
depends on the
polarizability
of the atomic assem-
blage. The highest refractive indices are found in min-
erals with metallic bonding, such as sulfides: amongst
the highest is galena, with a refractive index of 3.9.
Colour and absorption
Coupled substitution
A more complicated form of substitution is seen in pla-
gioclase feldspar. The series of compositions from
albite (NaAlSi
3
O
8
) to anorthite (CaAl
2
Si
2
O
8
) reflects
substitution in two sites at once. Substitution of Al
3+
for
Si
4+
in tetrahedral sites leaves a charge imbalance,
which is cancelled out by the
coupled substitution
of
Ca
2+
for Na
+
in the large cation sites. The overall subst-
itution is of CaAl for NaSi. It follows that the Ca con-
tent of plagioclase correlates with the Al content, and
the alkali (Na + K) content with Si. In the formula of
any accurate plagioclase analysis (calculated to 32 oxy-
gens), Si − (Na + K) will approximate to 8.0 (±0.1), and
Al − Ca to 4.0 (±0.1). These requirements provide
another measure of the quality of a plagioclase anal-
ysis (Exercise 8.3). Similar coupled substitutions occur
in pyroxenes: Na
+
Al
3+
for Ca
2+
Mg
2+
in diopside to give
jadeite (NaAlSi
2
O
6
), for example.
Another important optical property is absorption. A
mineral is
coloured
in transmitted light because certain
wavelength components in the visible spectrum are
more strongly absorbed than others, making the crys-
tal appear to have the
complementary colour
(Figure 8.4).
The region of absorption is generally a wavelength
band rather than specific sharp lines. The green colour
of olivine, for example, is due to absorption bands at
each end of the visible spectrum, which emphasize the
green wavelengths in the middle of the spectrum in the
light emerging from the crystal.
The absorption of light energy is related to electron
transitions between appropriately spaced energy levels
in the crystal. The quantum energy of visible light is
much smaller than that of X-rays (Chapter 6), and the
transitions concerned occur between valence energy
levels, either within an atom or between neighbouring
ions. One very important factor in the colour of minerals
Optical properties of crystals
Red
Crystal optics, a specialized subject for which several
excellent textbooks are available, is mostly beyond the
scope of this topic.
5
It is, however, relevant to take a
brief look at how the optical properties of a crystal
relate to its chemical bonding.
Yellow
Violet
Refractive index
Light, like any electromagnetic signal, consists of syn-
chronized oscillations of electric and magnetic fields
(Boxes 5.2 and 6.3). It is the electric vector that is
Green
Blue
Tu rquoise
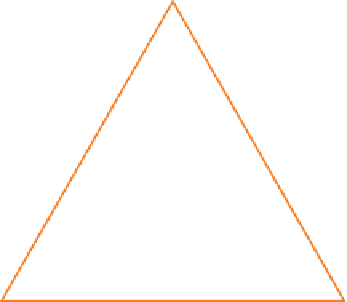
Figure 8.4
Colour triangle showing complementary
colours. Absorption of one colour will leave light relatively
enhanced in the colour on the opposite side of the figure.
5
A very simple introduction to crystal optics can be found at


Search WWH ::

Custom Search