Geology Reference
In-Depth Information
Box 8.5 Cation sites in pyroxenes and amphiboles
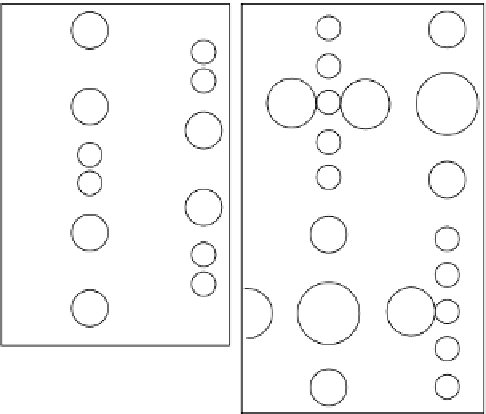
Figure 8.5.1a shows a simplified end-on view of the pyroxene
chains, indicating how they are stacked together, alter-
nately back-to-back and point-to-point. In addition to the
Z-sites (not shown) occupied by Si and a little al, there are
two types of cation site. Between two chains facing each
other point-to-point lie two octahedral sites, designated
M1, in which al
vi
('octahedral al'), Fe
2+
, Fe
3+
, Mg
2+
, Mn
2+
,
Cr
3+
and ti
4+
are accommodated. the other type of site
lies between pairs of chains whose tetrahedra face each
other base-to-base. they are called M2 sites and their
geometry varies according to the ions occupying them. In
the absence of Ca or Na, M2 is occupied by Mg
2+
, Fe
2+
and Mn
2+
(for example in enstatite, Mg
2
Si
2
O
6
), and has
an irregular 6-fold co-ordination. the chains stack together
in such a way as to produce an orthorhombic unit cell
(orthopyroxene). the substitution of larger ions like Ca
2+
or
Na
+
causes a change of M2 geometry to 8-fold co-ordination.
the presence of the larger ion disrupts the stacking, and
forces the structure to adopt a lower-symmetry (monoclinic)
structure, as in diopside (CaMgSi
2
O
6
).
Owing to the broader bands in the amphibole structure
(Figure 8.5.1b), there are three slightly different types of
octahedral site (two M1 sites, two M2 sites and one M3
site) in corresponding positions to the pyroxene M1 site.
these are the five 'C' sites in the formula given in the
main text. a larger site called M4 (or 'B' in the formula)
corresponds almost exactly to M2 in the pyroxenes,
accommodating small ions like Mg
2+
in 6-fold co-ordin-
ation, and larger ions like Ca
2+
and Na
+
in 8-fold co-ordin-
ation. the occupant of M4 plays the same role as M2 in
the pyroxenes in determining the symmetry of stacking:
Mg-rich amphiboles are commonly orthorhombic, whereas
all other compositions are monoclinic. there are two M4
('B') sites per formula unit.
the so-called a-site has no equivalent in pyroxenes. It
lies sandwiched between pairs of bands whose under-
sides face each other, associated with the hexagonal rings
that account for its large size. It is commonly unoccupied,
but may contain Na
+
or K
+
. the a-site lies opposite the
Oh-site, which also has no equivalent in the pyroxene
structure. Some sheet silicates exhibit similar architecture
(Box 8.2).
(a)
(b)
M2
M4
M2
M1
M1
M1
OH
-
OH
-
M3
A
M2
M2
M1
M1
M2
M4
M1
M2
M2
M4
M2
M1
M1
M1
M2
OH
-
OH
-
A
M3
M1
M4
M2
Figure 8.5.1
(a) Simplified view of the crystal structure
of a pyroxene. Double triangles represent the silicate
chain viewed end-on as in Figure 8.3a; M1 and M2
represent the two types of cation site (see text). (b)
Similar representation of the structure of an amphibole.
Groups of four triangles represent the silicate double
chains end-on as shown in Figure 8.3b. M1, M2 and M3
represent three types of octahedral site; M4 is a larger
site, and a (often partially filled) is larger still, nestling in
the ring structure of the double chain (Figure 8.3b).
Effects of cation substitution
nevertheless causes a slight expansion of the crystal
lattice: the
c
cell-dimension, for example, increases
from 0.5981 (Fo) to 0.6105 nm (Fa). The substitution of Fe
2+
for Mg
2+
gives rise to similar, continuous solid solution
series in garnets, pyroxenes, amphiboles and micas.
The effect is different when the substituting ion has a
different size. Replacing Mg
2+
in the pyroxene B-site
with the larger Ca
2+
ion forces the pyroxene to adopt a
In the olivine solid solution series extending from
the end-member forsterite (Fo = Mg
2
SiO
4
) to fayalite
(Fa = Fe
2
SiO
4
), Mg ions in the sites are progressively
replaced by Fe
2+
ions. This
substitution
of Fe
2+
for Mg
2+
is possible because the ions have the same charge
and similar size (Box 7.2). The larger radius of Fe
2+





























Search WWH ::

Custom Search