Geology Reference
In-Depth Information
Chain silicates
the most important mineral groups, the
pyroxenes
. The
chains are 'kinked' rather than linear because alternate
tetrahedra stick out in opposite directions. Each silicon
atom possesses two non-bridging oxygens (
p
= 2) and
shares two bridging ones, so that the composition of
the whole chain can be written (SiO
3
)
n
, where
n
is the
number of tetrahedra in the chain. All pyroxenes there-
fore have SiO
3
(or Si
2
O
6
) in their chemical formulae, as
for example in diopside CaMgSi
2
O
6
. The chains can be
stacked against each other in different ways, allowing
pyroxenes the potential to crystallize in both the
orthorhombic and monoclinic systems (Box 8.5).
The chains define the crystallographic
c
-axis in pyrox-
enes. Parallel to this run several prismatic cleavage
planes, such as the perfect {110} cleavage responsible for
the characteristic perpendicular cleavages seen in a
basal cross-section. These cleavages reflect the stronger
cohesion
within
each chain compared with the strength
of bonding
between
chains.
The sharing of two oxygen atoms by each SiO
4
group
produces
chains
of tetrahedra of indefinite length
(Figures 8.2 and 8.3a) that form the skeleton of one of
Figure 8.2
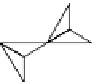
3D scale model showing the layout of a pyroxene
chain, with some upper oxygen anions removed to show
the tetrahedrally co-ordinated silicon atoms beneath.
(Photo: K. d'Souza).
Ring silicates
An obvious alternative to forming an infinite linear
chain is to link the ends of the chain into a ring. The
minerals beryl (Be
3
Al
2
Si
6
O
18
, Figure 8.1c), cordierite
(A1
3
Mg
2
Si
5
AlO
18
) and tourmaline are examples of ring
silicates, in which the basic structural element is a ring
of six SiO
4
tetrahedra. The simpler ring silicates have the
p
value (2) and Si:O ratio (1:3) of the pyroxenes (Table 8.1).
(a)
Pyroxene
(b)
Amphibole
Double-chain silicates
The
amphibole
structure can be regarded as a pyroxene
chain in which alternate tetrahedra share an oxygen
atom with one neighbouring chain. This produces a
double chain or band (Figure 8.3b), leading to a marked
prismatic, sometimes even fibrous, habit. Owing to
the wider double chains, the conspicuous prismatic
cleavages intersect at about 55°, compared with the 90°
characteristic of pyroxenes.
The double chain can be regarded as a row of hexag-
onal rings, which are not present in pyroxenes. These
accommodate additional anions, usually
hydroxyl
(OH
−
)
or fluoride (F
−
). Owing to the presence of these volatile
constituents - the main chemical distinction between
pyroxenes and amphiboles - the amphiboles are unstable
at high temperatures, decomposing into pyroxene and
vapour.
(c)
Sheet silicate
Figure 8.3
Simplified silicate structures in (a) pyroxene, (b)
amphibole and (c) sheet silicates, seen in plan and end-on.


































































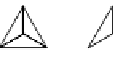































Search WWH ::

Custom Search