Geology Reference
In-Depth Information
z
x
y
s
z
x
y
p
x
p
z
p
y
z
z
z
x
x
x
y
y
y
d
z
2
d
x
2
-
y
2
d
xy
d
yz
d
zx
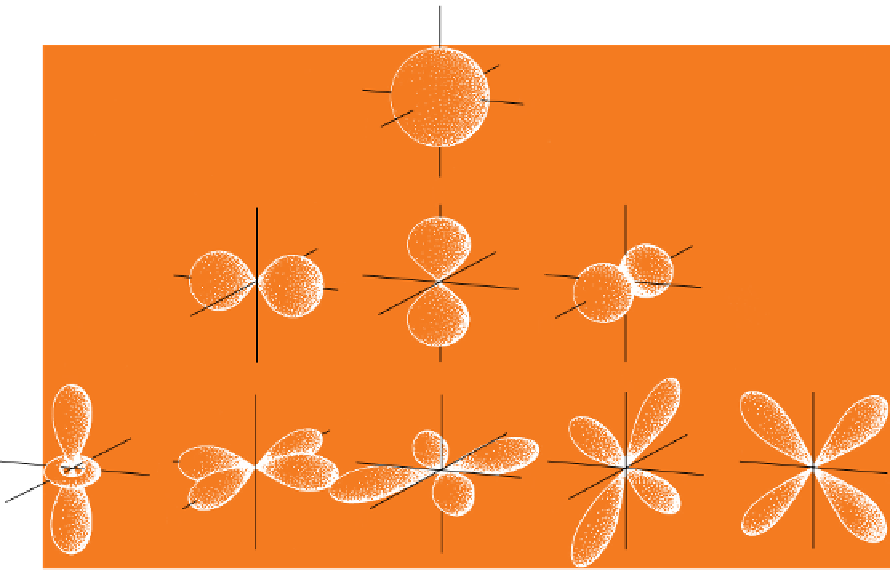
Figure 5.5
Simple 'balloon' diagrams showing where electron density is concentrated in s, p and d orbitals. Each balloon can
be considered as a three-dimensional contour of electron density.
Electron energy levels
the energy levels of electrons in different types of atom.
All of the energy levels have negative electron ener-
gies, indicating that an electron enjoys greater stability
within an atom than outside it.
Each box in Figure 5.6 represents an
orbital
.
Considered together, the orbitals resemble an irregular
set of 'pigeonholes' in energy space, offering the elec-
tron a variety of alternative accommodation in the
atom. The 1 s orbital has by far the lowest energy level,
indicating that in this state the electron is most firmly
bound to the nucleus. This interpretation is consistent
with the very small size of the 1 s orbital (Figure 5.3),
which confines the electron very closely to the nucleus,
where its electrostatic pull is strongest. This is the most
stable stationary state the electron can adopt in the
atom, the one in which its energy is minimized
(Chapter 1). When the electron resides in this orbital,
the hydrogen atom is said to be in its
ground state
.
The 2 s and the three 2p orbitals share the same
energy level, some distance up the energy scale.
In spite of their different spatial configurations,
Each stationary state of the Schrödinger equation pos-
seses a well-defined value of the total electron energy
E
(the sum of the electron's
potential
and
kinetic ener-
gies
). The energy of an electron in an atom is therefore
not free to vary continuously, but like the frequency of
a guitar string is
quantized
and has to conform to one of
these permitted
energy levels.
The Danish physicist
Niels Bohr had been the first to suspect this on empiri-
cal grounds in 1913, but the theoretical basis remained
obscure until Schrödinger's work showed it to be a
straightforward consequence of the stationary wave(s)
set up by electron(s) trapped in an atom.
The energy levels of the various orbitals are shown
in relation to each other (for the simplest example, the
hydrogen atom) in Figure 5.6. The zero on the energy
scale is defined as the energy of a 'free electron at rest',
one that does not belong to any atom (see caption) and
possesses no kinetic energy. This convention provides
a common baseline that allows us to compare directly


Search WWH ::

Custom Search