Geology Reference
In-Depth Information
the nucleus. Such solutions are called
s-orbitals
.
The
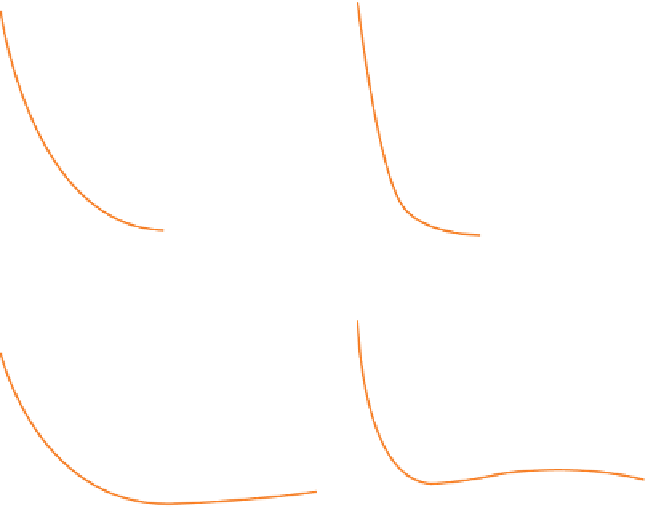
two simplest cases are shown in Figure 5.3. The upper
half shows what we may think of as the atomic coun-
terpart of the fundamental mode on a vibrating string
(Figure 5.2). In keeping with this interpretation, the
principal quantum number
n
is equal to 1 and the
number of nodes is zero. This orbital is designated '1 s'.
On the right-hand side of Figure 5.3 is an attempt to
show what a cross-section of this orbital would look
like. The electron density (represented by the density
of dots) is greatest immediately around the nucleus,
and decreases smoothly away from it with an expo-
nential-like profile. This diffuse outer fringe is com-
mon to all types of orbital, and in this respect
wave-mechanical waveforms differ significantly from
vibrating string harmonics, which of course terminate
abruptly at the end of the string. All atoms and ions,
therefore, have diffuse outer margins.
The lower part of Figure 5.3 illustrates a more
complex spherical orbital, designated 2 s, which
resembles a first harmonic:
n
has the value 2, and there
is a node-like feature where the wave function
ψ
and
therefore the electron density
ψ
2
are both zero. In three
dimensions, this is actually a spherical nodal surface,
separating a core of electron density from an outer
fringe. These two parts of the orbital together accom-
modate the same electron which, somewhat para-
doxically, distributes itself statistically between them.
Note that the electron density extends significantly
further from the nucleus than was the case for the 1 s
orbital (Figure 5.3).
Each of these orbitals is uniquely defined by the val-
ues of the two quantum numbers
n
(=1 or 2) and
l
(=0)
.
A series of progressively larger, and more complex,
spherical orbitals exists corresponding to values of
n
up to about 7, and these are distinguished as 3 s, 4 s and
so on, according to the value of
n
.
p-orbitals
The p-orbitals are a second class of solution (identified
by
l
having the value 1), in which electron density is
concentrated into two 'balloons' which stick out from
the nucleus in opposite directions (Figure 5.4). The lack
of spherical symmetry here requires the introduction of
arbitrary
x, y
and
z
axes, centred on the nucleus, in order
to specify the varying orientations of these balloons.
Radial variation of wave function
ψ
Radial variation of electron density
ψ
2
Electron density
cross-section
ψ
ψ
2
1s
r
r
2s
Node
Node
Figure 5.3
Alternative ways of visualizing s-orbitals. In the cross-section representations on the right, the density of dots
indicates the electron density.






Search WWH ::

Custom Search