Environmental Engineering Reference
In-Depth Information
O
NH
2
SO
3
H
N
N
N
SO
3
H
O
HN
NN
RB5
SO
3
H
Cl
DyP
NH
2
O
SO
3
H
OH
OH
+
H
N
H
N
HN
N
SO
3
H
O
N
N
(1)
SO
3
H
Cl
NH
2
H
N
H
N
H
2
N
N
SO
3
H
SO
3
H
+
NN
SO
3
H
Cl
o
-ABS
(2)
DyP
SO
3
H
N
N
N
H
N
N
H
2
N
H
2
N
N
SO
3
H
HO
3
S
(4)
NN
NN
SO
3
H
Cl
Cl
(3)
(3)
and/or
+
+
H
2
N
H
2
N
SO
3
H
SO
3
H
m
-ABS
p
-ABS
Fig. 16 Proposed pathway for the biotransformation of reactive blue 5 by DyPs. The presence of
products (1) and (2) was consistent with an oxidative ring-opening of the anthraquinone frame
generated by DyP, which appears in this case to behave as a hydrolase or oxygenase rather than a
peroxidase, although H
2
O
2
was indispensable for the reaction. The formation of compound (4) can
be explained by a reaction mechanism of a typical peroxidase leading to the formation of a radical
from o-ABS, which will be further involved in a spontaneous chemical reaction. Product (4) was
characterized by NMR and ESI-MS techniques and the formation of products (1), (2) and (3) was
supported by ESI-MS analysis of the
nal reaction mixtures (adapted from Sugano et al.
2009
)
aromatic amines that represent good oxidative substrates (Sousa et al.
2013
).
Therefore, azo dyes were reduced by PpAzoR under anaerobic conditions and after
24 h of reaction, CotA-laccase was added with agitation. Interestingly, this
sequential enzymatic procedure resulted not only in 100 % decolorization of all azo
dyes tested, but also in 50
cation of dye-products that exhibited the
highest initial toxicity (Fig.
17
) (Mendes et al.
2011a
).
-
95 % detoxi





































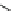




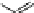



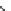





















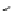




















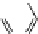













































Search WWH ::

Custom Search