Environmental Engineering Reference
In-Depth Information
Table 1 Activities of PpDyP and BsDyP as compared to PpAzoR and CotA, using 2 mM of
anthraquinonic (disperse blue 1, reactive blue 5, acid blue 62 and reactive blue 19) or azo (mordant
black 9, acid black 194 and acid yellow 49) dyes as substrate (Santos et al.
2014
)
Substrates
V
max
(U mg
−
1
)
PpDyP
BsDyP
PpAzoR
CotA
AQ dyes
Disperse blue 1
nd
0.6
±
0.04
10
±
3
3
±
0.1
0.3
±
0.01
Reactive blue 5
nd
11
±
1
9
±
1
Acid blue 62
nd
1.3
±
0.9
9
±
1
10
±
0.1
Reactive blue 19
nd
nd
9
±
2
5
±
0.2
Azo dyes
Mordant black 9
2
±
0.1
1
±
0.3
26
±
2
4
±
0.1
Acid black 194
3
±
0.4
0.9
±
0.2
12
±
2
2
±
0.1
Acid yellow 49
2
±
0.3
2
±
0.2
10
±
1
3
±
0.2
nd not detected
120
BsDyp
PpDyp
100
80
60
40
20
0
AQ dyes
Azo dyes
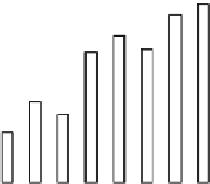
Fig. 14 Dye decolorization by the enzymes PpDyP (black bars) and BsDyP (white bars).
Decolorization was measured by HPLC after 24 h of reaction (adapted from Santos et al.
2014
)
or phenolic substrates tested, than the Bacillus enzyme. Moreover, PpDyP is able to
oxidise the high redox non-phenolic veratryl alcohol compound (1.4 V) in the
absence of redox mediators as DyPB from R. jostii and DyPs from C-D subfamilies













































































































































































Search WWH ::

Custom Search