Environmental Engineering Reference
In-Depth Information
1
N
OH
N
HO
SOG
NADPH
NADP
+
HN
OH
NH
HO
NADPH
2
NADP
+
+
NH
2
H
2
N
OH
HO
(
2
)
(
1
)
0
5
10
15
20
25
30
Retention time (min)
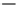
Fig. 10 Proposed mechanisms for the biotransformation of the azo dye Sudan orange G by
PpAzoR and HPLC chromatograms of the reaction mixture after 24 h of reaction with (thin line)or
without (thick line) PpAzoR. Products of the reaction were identi
ed, in comparison to the
standards: (1) aniline, (2) 4-aminoresorcinol
Fig. 11 Inhibitory effects of
intact dyes over
Saccharomyces cerevisiae
(dark bars) and
Caenorhabditis elegans
(dashed bars) (adapted from
Mendes et al.
2011a
)
120
100
80
60
40
20
0
DB1
AB194
DB38
RB5
RY145
DR80
Azo dyes
However, for the majority of the other dyes tested, the enzymatic products
present a higher toxicity than intact dyes themselves, as assessed by the S. cere-
visiae system, exhibiting 2 to 4-fold higher toxicity than intact dyes (Fig.
12
)
(Mendes et al.
2011a
).
3.3 Engineering of PpAzoR for Improved Thermal Stability
PpAzoR broad substrate speci
city makes it attractive for bioremediation processes,
but its low thermal stability (half life of 13 min at 50
C) impairs its full potential
for environmental related applications. Thermal stability is a critical property, as it
°































































































































































































































































Search WWH ::

Custom Search