Environmental Engineering Reference
In-Depth Information
Wesenberg et al.
2003
). This enzyme is basic in nature having an isoelectric point
between 3 and 5 depending on the isoform (Leonowicz et al.
2001
). It essentially
requires hydrogen peroxide (H
2
O
2
) to catalyze a reaction that occurs through a
cycle. It has high redox potential, which facilitates the LiP enzyme to directly
oxidize non-phenolic lignin units (Sarkar et al.
1997
). LiP abstracts single electron
from the aromatic rings of aromatic compound, leading to the formation of a cation
radical and subsequent cleavage reactions (Zheng and Obbard
2002
). A charac-
teristic of LiP, which is also shared by non-ligninolytic peroxidases, is its relative
unspeci
city for substrates, such as phenolic compounds and dyes (Martinez
2002
).
During oxidation of aromatic ring of non-phenolic compounds, aromatic cations are
formed by LiP and phenoxy radicals by the oxidation of phenolic substrates by
peroxidases (Kersten et al.
1985
; Martinez
2002
).
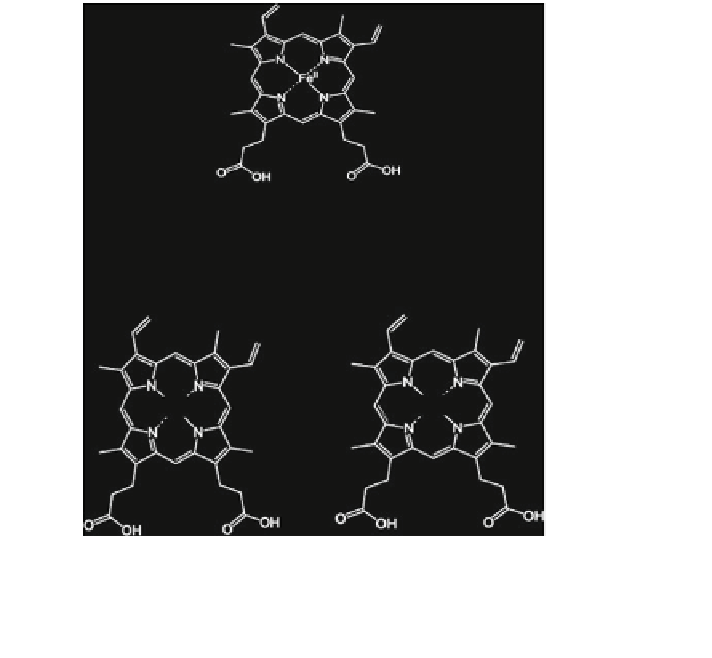
The native enzyme having heme group (ferric form) at the active site is oxidized
by H
2
O
2
with two electrons to compound I as re
ected in Fig.
2
. One electron is
abstracted from ferric [Fe(III)] iron to form ferryl [Fe(IV)], while the second
electron is removed from the porphyrin ring to form a porphyrin cation radical
R
.
+ H
2
O
Inactivation
H
2
O
2
Native Heme
H
2
O
RH
Compound III
H
2
O
H
2
O
2
Fe(IV)O
Fe(IV)O
Compound I
Compound II
RH
R
Fig. 2 Catalytic cycle of heme-containing peroxidases (modi
ed after Torres et al.
2003
)












Search WWH ::

Custom Search