Environmental Engineering Reference
In-Depth Information
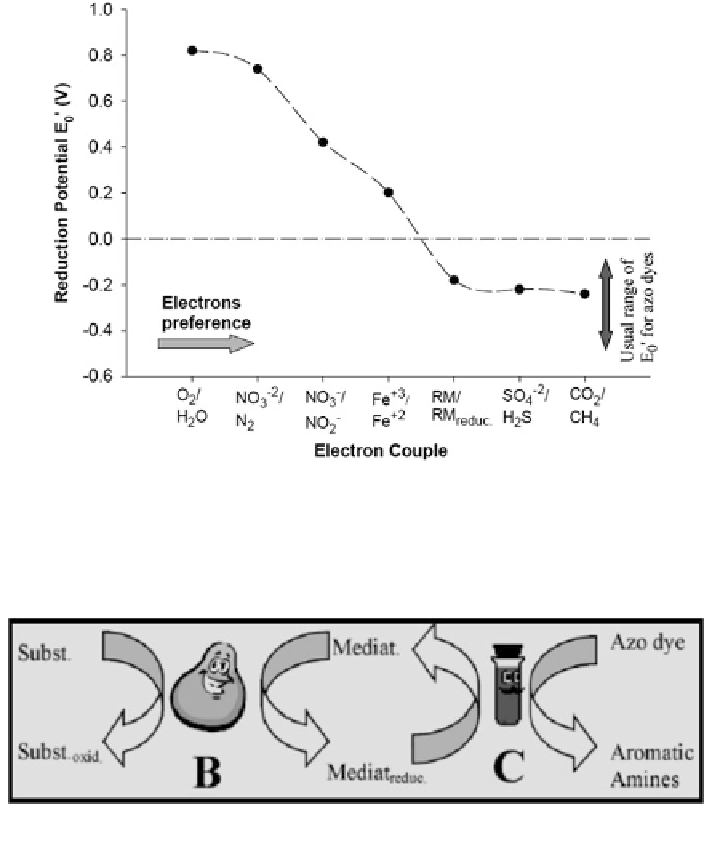
Fig. 4 Electron ow preference as a function of the different electron couples (Dubin and Wright
1975
; Cervantes
2002
; Madigan et al.
2003
; Dos Santos
2005
). RM and RM
reduc
are the oxidized
and the reduced forms of the redox mediator, respectively
Fig. 5 Schematic for indirect azo dye reduction. B and C are the biological and chemical steps,
respectively
electron donor (van der Zee et al.
2003
). Unfortunately, the standard redox potential
(E
′
0
) for most of azo dyes is unknown, however, this information can be obtained
by using polarography. In a screening of redox potential values for different azo
dyes, it was found that E0 values are generally between -0.430 and -0.180 V (Dubin
and Wright
1975
). Rau et al. (
2002a
,
b
) stated that the NAD(P)H co-factor, which
had the lowest E
0.320 V, set the limits of redox mediators application.
The reason for this is that mediators with a more negative E0 value will not be
reduced by the cells, and mediators with E
′
0
value of
−
′
0 greater than
−
0.05 V will not
Search WWH ::

Custom Search