Environmental Engineering Reference
In-Depth Information
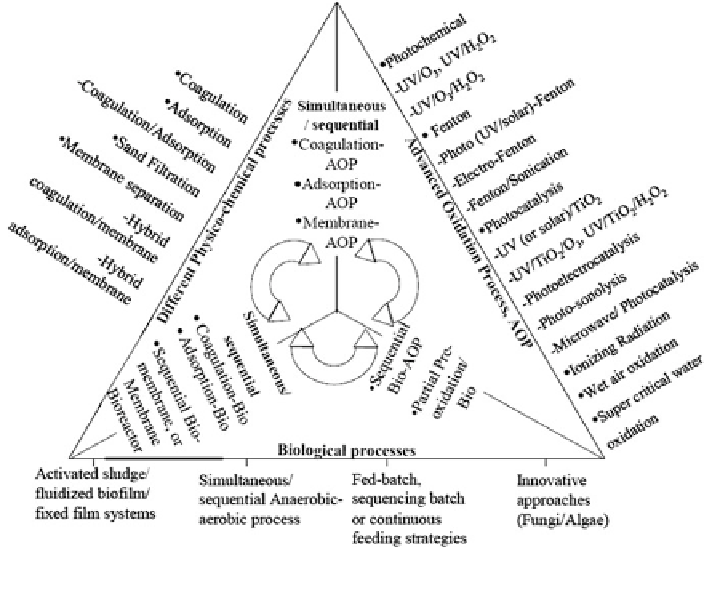
Fig. 1 Simplied representation of broad spectrum of combinations proposed by many workers
NADH or NAD(P)H donor (Madigan et al.
2003
). Although azo dyes are aromatic
compounds, but their substituents containing mainly nitro and sulfonic groups make
them recalcitrant to aerobic bacterial degradation (Claus et al.
2002
). This may be
related either to the electron-withdrawing nature of the azo bond and their resistance
to oxygenases attack, or oxygen is a more effective electron acceptor, as it has more
preference for reducing equivalents than the azo dye (Chung et al.
1992
; Knack-
muss
1996
). However, in the presence of speci
c oxygen-catalyzed enzymes, called
azo reductases, some aerobic bacteria are able to reduce azo compounds and pro-
duce aromatic amines (Stolz
2001
). Presence of aerobic azo reductases was found in
Pseudomonas species strains K22 and KF46 (Zimmermann et al.
1982
,
1984
).
These enzymes, after puri
cation, characterization and comparison, were shown to
be
avin-free. The aerobic azo reductases were able to use both NAD(P)H and
NADH as co-factors and reductively cleaved not only the carboxylated growth
substrates of the bacteria, but also sulfonated structural analogues. Recently, Blu-
mel and Stolz (
2003
) cloned and characterized the genetic code of the aerobic azo
reductase from Pagmentiphaga kullae K24. This strain was able to grow with the
carboxylated azo compound 1-(4
-carboxyphenylazo)-4-naphtol as a sole source of
carbon and energy. Furthermore, the gene encoded a protein with a molecular
weight of 20,557 Da, having a conserved putative NAD(P)H-binding site in the
amino-terminal region.
′
Search WWH ::

Custom Search