Environmental Engineering Reference
In-Depth Information
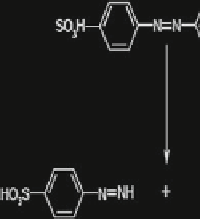
Methyl Orange
Asymmetric Cleavage by
Purified Laccase
p
- phenyldiazine sulphonic acid
p
-hydroxy N,N'dimethyl benzene
Fig. 11 Proposed degradation pathway for Methyl Orange by puri
ed laccase
7.3 Lignin Peroxidase Mediated Dye Decolorization
The lignin peroxidase puri
ed from Bacillus laterosporus was decolorizes Methyl
Orange (Gomare et al.
2008
). The biodegradation pathway of Methyl Orange was
shown in Fig.
12
.
In degradation pathway, Methyl Orange
rstly forms cyclo-2, 5-ene-1-one rad-
icals with the removal of sulfate ion, N, N di methylamine and a nitrogen molecule,
where these two radicals condense to produce 1, 1
′
-dicyclohex-2, 5-ene-4-one
moiety. Its one of the cyclohexene rings undergoes
-cleavage to form 4-substituted
hexanoic acid, which on the removal of methyl radical undergoes cyclization to
produce 4-cyclohexenone lactone cation. This substituted lactone cation
α
nally
forms a stable molecule of p-isopropanal-2,5-cyclohexa-dienone with the elimina-
tion of iminium moiety (Gomare et al.
2008
).
7.4 Veratrol Alcohol Oxidase Mediated Dye Decolorization
The veratrole alcohol oxidase was puri
ed from Comomonas sp. UVS and assessed
fordye decolorization (Jadhav et al.
2009
). Oxidative cleavage of Red HE7B
(RRHE7B) yielded an unknown product. This product further undergoes desulfo-
nation to give naphthalene-1,2,5-triol (Fig.
13
). The use of puri
ed enzyme sug-
gested their direct involvement for dye decolorization.
8 Toxicity of Azo Dyes and Its Degradation Metabolites
The wastewater released from the textile industries after the treatment is sometime
used for irrigation purpose in the agricultural
elds (Paul et al.
2012
). Hence, the
toxicity assessment for the seed germination and plant growth of treated textile

Search WWH ::

Custom Search