Environmental Engineering Reference
In-Depth Information
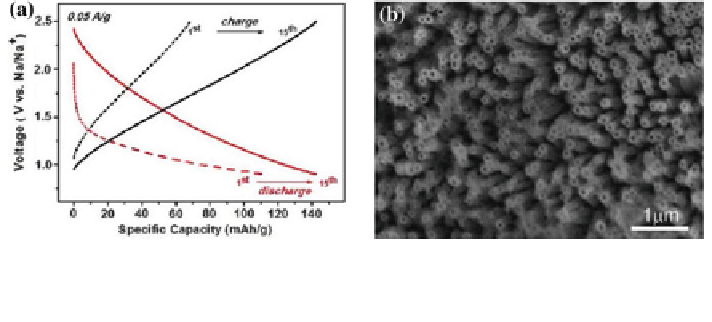
Fig. 27 a Charge/discharge galvanostatic curves of amorphous 80 nm I.D. TiO
2
NT in Na half
cell (red for discharge and black for charge) cycled between 2.5 and 0.9 V versus Na/Na
+
at
0.05A g
-1
. b SEM top-view images of TiO
2
NT electrodes [
74
]
consists of zigzag layers of Ti-O octahedra with Na ions in the interlayer spacing
[
77
]. Additional Na ions (2 Na ions per formular unit) can insert into the interlayer
space.
The
Na
2
Ti
3
O
7
electrode
could
reversibly
deliver
a
capacity
of
200 mAh g
-1
at an average potential of 0.3 V and with good cycling stability.
5 Summary and Outlook
Although along with the development of Li intercalation materials, their Na
analogs have also been investigated, the performance of Na intercalation materials
is far inferior to the lithium counterparts, the energy density of the thus constructed
Na-ion batteries then is far below that of Li-ion batteries. Moreover, some research
on Na intercalation reactions focus only on the superficial exploratory study, deep
consideration of materials and systems are bare. Time enters in the twenty-first
century, and due to the shortage of fossil fuel and the worsening of environmental
pollution, the development of renewable energy sources and electric vehicles has
drawn more and more attention. Li-ion batteries that have occupied the portable
electronic product market are considered as the ideal system for electric vehicle
propulsion and renewable electric power storage. However, due to the concern of
insufficient Li reserves, scientists again begin to show interest in Na-ion battery
systems, which have no resource constraints. In the last 3 years, a wide range of
new types of Na intercalation materials have been proposed and deeply investi-
gated. The electrochemical performance of several materials was close to that of
Li-ion batteries. For example, P2-Na
0.67
Fe
0.5
Mn
0.5
O
2
cathode material can reach
an energy density of 520 Wh kg
-1
, which is comparable to that of LiFePO
4
(about
530 Wh kg
-1
) and slightly higher than LiMn
2
O
4
(about 450 Wh kg
-1
). Rhom-
bohedral Na
1.72
MnFe(CN)
6
has a reversible capacity of 134 mAh g
-1
with a high
potential plateau of 3.4 V, very close to that of LiFePO
4
(140 mAh g
-1
and 3.4 V
potential plateau). For the anode, hard carbon with hollow nanowire structure can
Search WWH ::

Custom Search