Environmental Engineering Reference
In-Depth Information
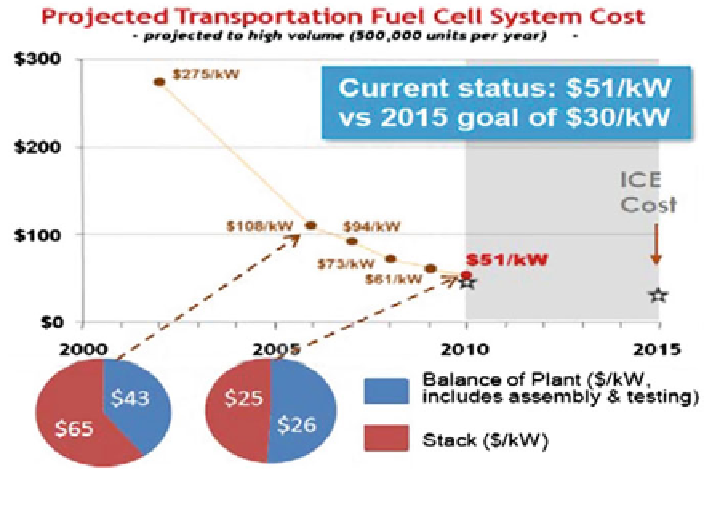
Fig. 1
DOE projected transportation fuel cell system cost (adapted from Ref. [
26
])
normally in the temperature range of 30-100 C under humidified environment
although PEMs that can operate up to 200 C are also available. The easy start-up
and flexible design has attracted interests in both stationary and portable appli-
cations [
4
,
18
,
19
]. In fact, it represents one potential case for reducing the green
house gas emission from a renewable energy point of view especially if hydrogen
is produced by solar technologies without any carbon footprint [
20
]. PEMFCs also
have higher practical efficiencies over commercial engines especially if combined
heat-power generation (cogent) is targeted [
21
].
A state-of-the-art PEMFC accordingly has five critical components: (i) Pt
electrocatalyst, (ii) catalyst support carbon, (iii) gas diffusion layer (GDL) or
backing layer, (iv) bipolar plates and (v) polymer electrolyte membranes. For the
successful operation of PEMFCs, the reactant H
2
and O
2
must reach the catalyst
site where the electrochemical reactions take place and the product water has to be
expelled from the catalyst site to prevent water clogging and better access of
reactant gases to the electrocatalyst. Similarly, the generated protons at the
electocatalyst sites must reach the cathode through PEM while the electrons need
to reach the cathode through an external circuit, where it does the useful work, to
react with oxygen and protons to form water. Hence, the effective formation of
triple-phase
boundary
(TPB)
(reactant
gases,
electrocatalyst
and
electrolyte
membrane) is an important criterion for successful PEMFC operation.
Despite many advantages, PEMFCs still have many challenges owing to the
availability of critical materials and processes to effectively address the lack of
commercialization aspects [
22
,
23
]. For example, the use of Pt as electrocatalyst,
Search WWH ::

Custom Search