Environmental Engineering Reference
In-Depth Information
(e)
(a)
800
600
400
200
2D Graph 1
0
0 min
5 min
10 min
30 min
60 min
90 min
120 min
150 min
180 min
Pd NPs
-200
4
-400
0
(b)
-600
-4
-8
-800
Pd NPs
-12
-1000
-.4
0.0
.4
.8
1.2
-1200
-.4
-.2
0.0
.2
.4
.6
.8
1.0
1.2
1.4
1.6
E (V vs Ag/AgCl)
(f)
(c)
1000
800
600
400
(d)
200
Pd NPs
0
0
20
40
60
80
100
120
140
160
180
200
t
(min)
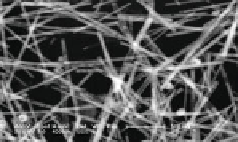
Fig. 26 Scanning electron microscopy (SEM) images of the PdAg nanotubes synthesized with
different galvanic reaction times: 10 min (a); 90 min (b); 150 min (c) and 180 min (d). e Cyclic
voltammograms of the PdAg nanotubes obtained at different galvanic reaction times and Pd
nanoparticles in 0.1 M HClO
4
electrolyte solution. Potential scan rate 0.1 V s
-1
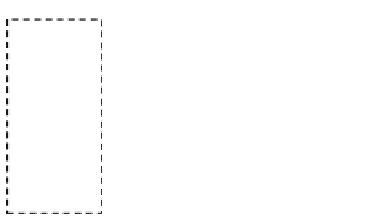
. For clarity, the
CV from Pd nanoparticles is shown in the inset. The dashed frame shows the hydrogen
adsorption-desorption region obtained on the PdAg nanotubes. f The total charge (Q
H
)of
hydrogen adsorption and absorption on the PdAg nanotubes dependent on galvanic reaction time.
Reprinted from Ref. [
125
] with permission by the Royal Society of Chemistry
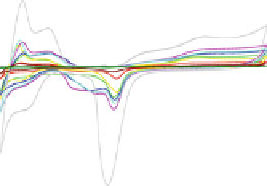
CVs (Fig.
26
e) of the synthesized PdAg nanotubes in a N
2
saturated 0.1 M HClO
4
aqueous solution, there is obvious hydrogen adsorption/desorption in the potential
range of -0.25 to +0.1 V. From the dependence of Q
H
on reaction time shown in
Fig.
26
f, it can be seen clearly that the hydrogen storage ability depends strongly
on the composition of the PdAg nanostructured materials. The PdAg nanotubes
with 15 % Pd possess the highest capacity for hydrogen absorption, which is over
200 times higher than that of pure Pd nanoparticles. Such significantly enhanced
ability for hydrogen storage can be ascribed to the special tubular structures and
alloying of Pd and Ag around the walls of the nanostructures. This work suggests
that 1D Pd-based alloy nanotubes with low Pd content might represent a unique
class of low-cost materials for efficient hydrogen storage.













































































Search WWH ::

Custom Search