Environmental Engineering Reference
In-Depth Information
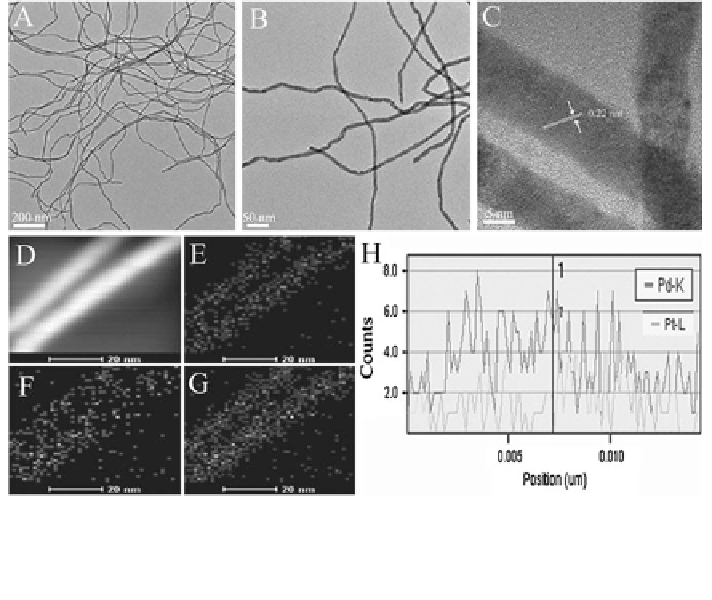
Fig. 16 TEM (a, b) and HRTEM (c) images of the obtained Pd
80
Pt
20
nanowires. HAADF-
STEM-EDS mapping images (d-g)ofPd
80
Pt
20
nanowires. The cross-sectional compositional line
profiles of individual Pd
80
Pt
20
nanowire (h). e Pd-K; f Pt-L; g Pd-K + Pt-L. Reprinted from
Ref. [
64
] with permission by Wiley-VCH
PdPt ANWs exhibit significantly enhanced activity and stability toward ethanol
oxidation in alkaline medium. Figure
16
a and b show the TEM images of the
Pd
80
Pt
20
ANWs templated from pre-synthesized Te NWs under different magni-
fications, clearly indicating the formation of uniform nanowires with high aspect
ratios, an average diameter of 10.8 nm, and lengths up to tens of micrometers.
HRTEM and elemental mapping measurements (Fig.
16
c-h) revealed that both Pd
and Pt elements are homogeneously distributed without significant segregation of
each component. Electrochemical experiments are then performed to evaluate the
catalytic activity of the PdPt ANWs toward alcohol oxidation. As shown in
Fig.
17
a, for the PdPt ANWs with different compositions, the Pd
45
Pt
55
ANWs
displays the highest activity toward ethanol oxidation in terms of onset potential
and peak current. Based on the CV comparison in Fig.
17
b, the mass activities on
the Pd
45
Pt
55
ANWs modified electrode is about 1.2 and 1.8 times of those on the
Pd NWs and Pt nanotubes electrodes for ethanol oxidation. Moreover, the Pd
45
Pt
55
ANWs also show a higher activity than commercial Pd/C electrocatalyst for eth-
anol oxidation. From the current-time curves recorded at -0.2 V shown in
Fig.
17
c, the Pd
45
Pt
55
ANWs exhibit the highest catalytic stability among the
studied electrocatalysts. On the other hand, the as-prepared 1D Pd
45
Pt
55
catalyst
also
shows
higher
electrocatalytic
activity
and
stability
compared
to
the
Search WWH ::

Custom Search