Environmental Engineering Reference
In-Depth Information
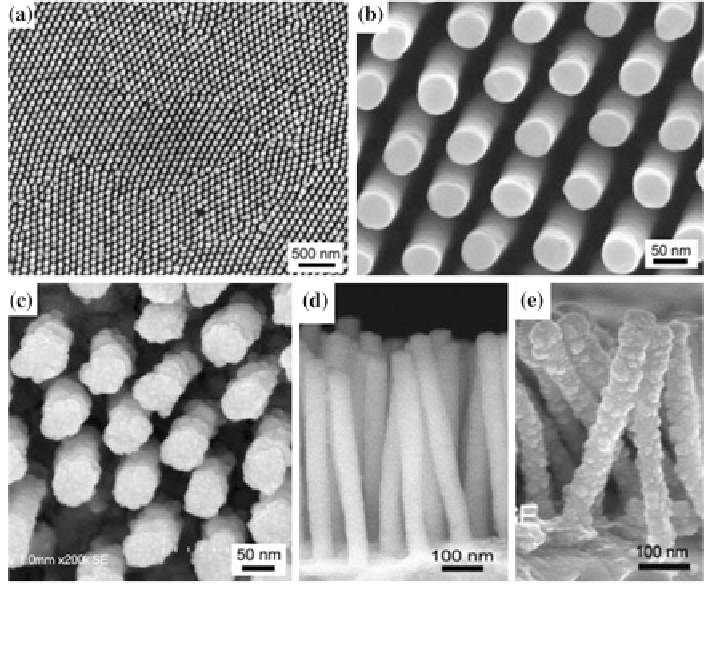
Fig. 9 SEM micrographs of a and b surface of Pd NWAs, c surface of Pd/Pt core-shell NWAs,
d cross-section of Pd NWAs, and e cross-section of Pd/Pt core-shell NWAs. Reprinted from
Ref. [
72
] with permission by Elsevier
Earlier studies have shown that 1D NWs have a strong interaction with carbon
supports and are less vulnerable than conventional nanoparticles to dissolution,
Ostwald ripening, and aggregation in strong acidic electrocatalytic conditions
[
71
,
103
]. Different from the bimetallic Pd-based alloys, Guo et al. [
104
] recently
synthesized ultrathin (2.5 nm) trimetallic FePtPd alloy nanowires (NWs) with
tunable compositions and controlled length (less than 100 nm). These FePtPd
NWs exhibited composition-dependent catalytic activity and stability for methanol
oxidation reaction. As shown in Fig.
11
a, the as-prepared Fe
28
Pt
38
Pd
34
NWs
exhibit the highest catalytic activity for methanol oxidation with the mass current
density of 488.7 mA mg
P
-1
and peak potential decreased from 0.665 V (vs. Ag/
AgCl) obtained on Pt nanoparticle catalysts to 0.614 V. More interestingly, the
Fe
28
Pt
38
Pd
34
displayed enhanced electrochemical stability with the mass current
density (98.1 mA mg
P
-1
) after i-t test for 2 h at 0.4 V (Fig.
11
b). Figure
11
c, d
indicates that there was almost no noticeable morphology change before and after
i-t tests, whereas the Pt nanoparticles experienced substantial aggregation. The
authors attributed the enhanced stability of NWs versus NPs to the stronger NW
interactions with carbon support and/or by better NW structure stability, which
Search WWH ::

Custom Search