Environmental Engineering Reference
In-Depth Information
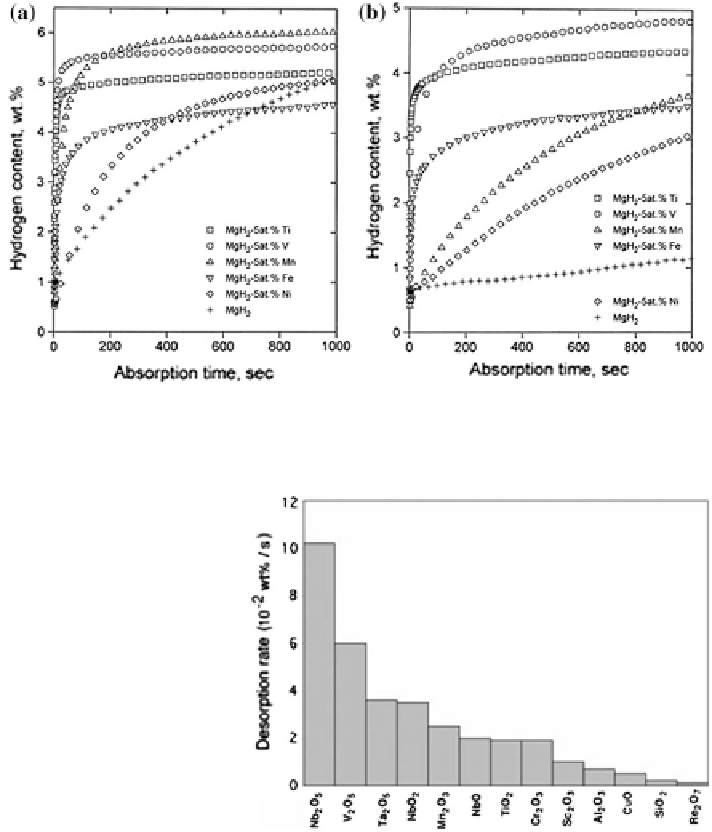
Fig. 12 Hydrogen adsorption curve of Mg-TM composites at a 273 K and b 373 K. Adapted
with permission from Liang 1999
Fig. 13 Catalytic effect of
different transition metal
oxides on the hydrogen
desorption reaction rate of
magnesium hydride at
T = 300 C. Reaction rates
were calculated between 20
and 80 % of the respective
maximum capacity. Adapted
with permission from
Barkhordarian 2006
Around the same time, Hanada et al. also performed kinetic studies on nano-
composites of MgH
2
with iron, cobalt, copper, nickel, and Nb
2
O
5
[
80
,
93
-
95
].
They found that each of these catalysts improves kinetics, with nickel and Nb
2
O
5
performing the best at 2 and 1 mol%, respectively. Using the JMA equation to
model their kinetics and TDMS, they were able to effectively apply the Kissinger
method [
76
] to estimate the activation energies to be 323 ± 40, 94 ± 3, and
71 ± 3 kJ molH
2
-1
for the non-catalyzed Mg-H
2
, nickel-catalyzed, and Nb
2
O
5
catalyzed hydrogen desorption reaction, respectively. The difference in the acti-
vation energy of the pure MgH
2
desorption process compared to that found by
Liang et al. [
5
] highlights the difficulty in comparing the capabilities of different
catalysts to improve sorption kinetics. As with others, Hanada et al. conclude the
Search WWH ::

Custom Search