Environmental Engineering Reference
In-Depth Information
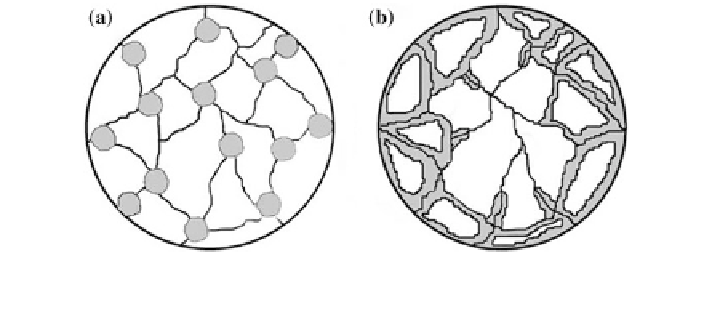
Fig. 11 Schematic picture of phase growth according to a JMA and b CV models. The dark
areas represent the transformed phase. Reproduced with permission from Barkhordarian 2006
method calls for data correction due to the kinetic behavior being dependent on
both the temperature and pressure. It has been shown that as the change in pressure
is measured, which is how the data is collected, the driving force for the exchange
of hydrogen is altered [
89
]. This driving force is dependent on the equilibrium
pressure, which is defined as the pressure at which the system must be above or
below, in order for hydrogenation or dehydrogenation to proceed. The change in
driving force is not significant if the process is performed with a pressure con-
siderably higher or lower than the equilibrium pressure, depending on which
process is monitored. However, if this is not the case, the deviation of the driving
force can be corrected by plotting F(a)/F(P) versus t, where F(P) is the pressure
dependence function [
68
,
89
]. Common F(P) functions can be found in Table
3
[
89
]. Proper methods for determining the pressure dependence function can be
found in the work of Sanchez et al. [
90
]. P is the measured pressure at time t, P
br
is
the reaction bed pressure (described in detail in reference) [
89
], and P
eq
is the
equilibrium pressure of the Mg +H
2 ?
MgH
2
reaction. P
eq
values can be deter-
mined by performing the pressure sweep method [
91
] or estimated by utilizing
values reported in the literature [
68
,
92
]. Again, the slope of the new plot, F(a)/
F(P) versus t, is the kinetic rate constant.
Quantitative characterization of the sorption kinetics of nanoscaled materials
has proven difficult if not unattainable. However, with the methods that have been
developed thus far, at least qualitative comparison of catalytic ability is possible.
As will be evident in the next section, the lack of activation energy values reported
in the literature makes the comparison of hydrogenation and dehydrogenation
kinetics elusive, and with respect to mechanism, arduous to fully understand.
4 Doping Studies
As mentioned earlier in
Sect. 2.2
, in addition to reducing the grain size of mag-
nesium-based hydrogen storage materials, researchers have attempted alloying or
''doping'' Mg nanostructures with small amounts, less than *10 %, of various
Search WWH ::

Custom Search