Environmental Engineering Reference
In-Depth Information
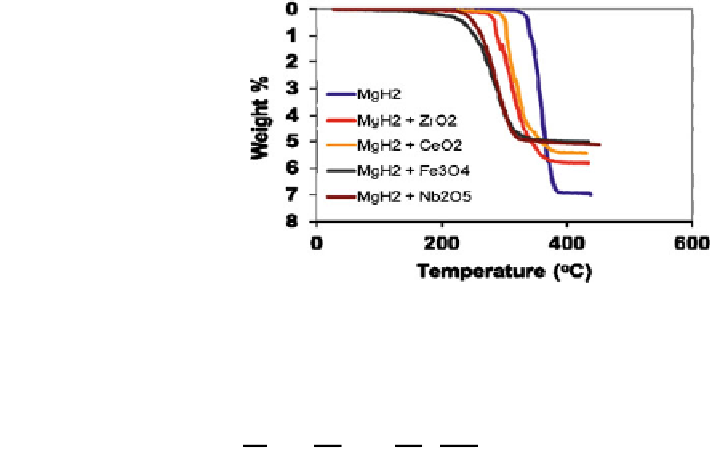
Fig. 7 TGA measurements
on magnesium hydride with
and without different
nanocomposites. Reproduced
with permission from Sabitu
2012
"
#
ln b ¼
0
:
457E
a
RT
R
AE
a
da
f
ðÞ
a
Z
2
:
315
log
ð
1
Þ
0
"
#
T
2
¼
E
a
b
E
a
RA
a
da
f
ðÞ
Z
ln
RT
ln
ð
2
Þ
0
where b is the ramp rate, E
a
is the activation energy, R is the gas constant, and A is
the pre-exponential factor of the Arrhenius equation. The kinetic function, f( a),is
dependent on the fraction of converted material at a given time over the maximum
converted material [
73
]. These two variables will be discussed further in later
sections. Determining the activation energy with Eq. (
1
) is known as the Ozawa-
Flynn-Wall (OFW) method [
74
,
75
]. Alternatively, the Kissinger-Akahira-Sunose
(KAS) method can be used to find the activation energy with Eq. (
2
)[
76
]. By
varying the ramp rates of several TGA or DSC scans, a linear relationship can be
observed by plotting ln(b) versus 1/T or ln(b/T
2
) versus 1/T, depending on which
method is used. The slope of the plot can then be used in correlation with Eq. (
1
)
or (
2
) to calculate the activation energy. Different data sets are obtained in TGA
than with DSC. As can be seen in the TGA data shown in Fig.
9
[
74
], different
fractions of completion can be chosen prior to analysis. From here, the different
values of ramp rates and corresponding temperatures can be used to form, for the
purpose of repetition, several linear plots. Alternately with DSC, the various ramp
rates are plotted with the temperature at which the reaction rate is greatest (Fig.
8
)
[
40
]. There has been much debate about which method, OFW or KAS, should be
utilized due to the accuracy of the calculated value. However, the discrepancies
between the methods are small enough that a semiquantitative comparison of
activation energies can be done to compare the kinetics [
73
,
77
]. The advantage of
using these methods to study kinetics of desorption is that an activation energy
may be calculated without the difficulty of fitting the desorption curve to an
appropriate kinetic function, f(a). However, this is not the case for the PCI or GCI
methods, which have the added advantage of being able to study both the hydrogen
adsorption and desorption kinetics.

Search WWH ::

Custom Search