Environmental Engineering Reference
In-Depth Information
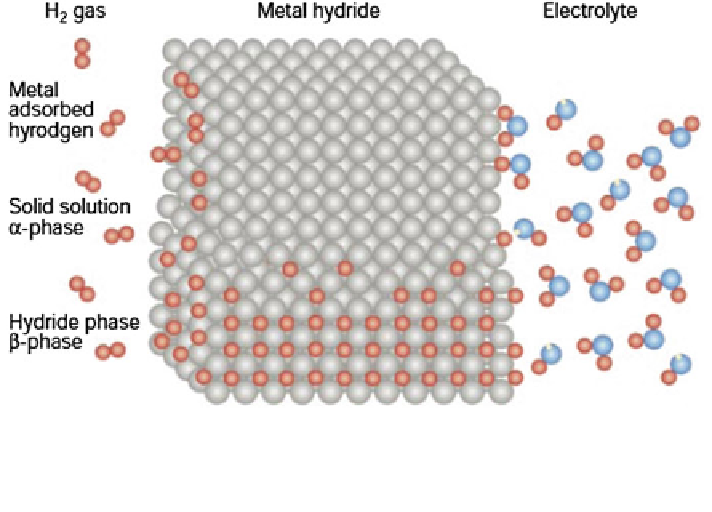
Fig. 2 Schematic model of a metal structure with H atoms in the interstices between the metal
atoms, and H
2
molecules at the surface. Hydrogen atoms are from physisorbed hydrogen
molecules on the left-hand side and from the dissociation of water molecules on the right-hand
side. Reproduced with permission from Schlapbach 2001
inhomogeneous, poorly crystalline, and with ill-defined particle size. An important
but unanswered question is still whether or not the increased kinetics observed for
nanostructured materials are due purely to increased surface area, or if grain
boundaries and trace impurities or catalysts are important for the hydridation
reaction.
This chapter will provide an overview of the synthesis of nanostructured
magnesium-based materials, the resulting kinetics of these materials (and how
these kinetics are currently modeled) as well as theoretical predictions of what can
be obtained using different approaches toward magnesium. For the purposes of
brevity, we have focused on nanostructured magnesium, as well as doped mag-
nesium, but we have not included a discussion of composite materials where
magnesium is not the major component. The references contained herein are meant
to be as comprehensive as possible, to provide the reader with reasonable
resources with which to read further about this fascinating material.
2 Synthesis
In order to optimize the performance of any material, a deep understanding of the
effects of the synthesis of the material on the resulting properties is imperative. For
hydrogen storage materials in particular, it is important to fully understand the
sorption kinetics for these systems and how the kinetics depend on the quality of
Search WWH ::

Custom Search