Environmental Engineering Reference
In-Depth Information
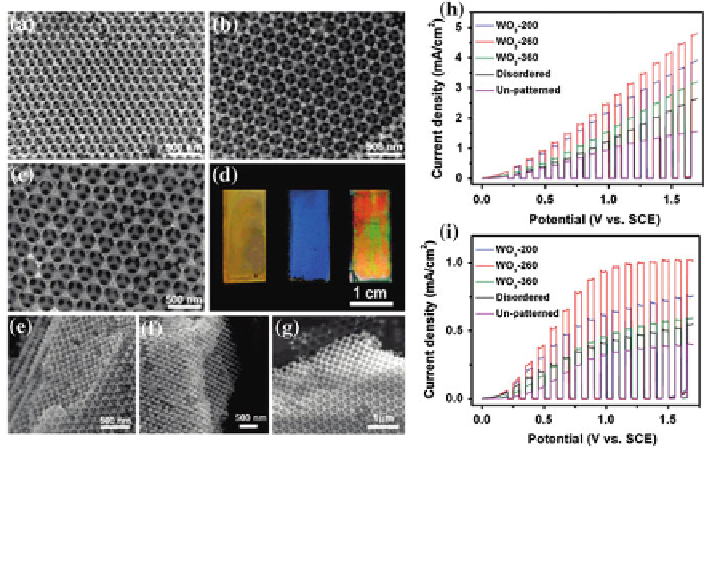
Fig. 9 a-c SEM top view of WO
3
inverse opals with 200, 260 and 360 nm pore size.
d photograph of the inverse opal WO
3
-200, WO
3
-260 and WO
3-
360. e-g SEM images of the
cross-section view of WO
3
-200, WO
3
-260 and WO
3-
360, respectively. h-i linear sweep
voltammograms of all the WO
3
electrodes under chopped UV light illumination and visible light
illumination. Reproduced with permission from [
6
]
3.3 a-Fe
2
O
3
Nanomaterials for PEC Water Oxidation
Hematite (a-Fe
2
O
3
), as one of the most prevalent metal oxides on earth, has
attracted extensive attentions for PEC water splitting, due to its favorable band-
gap, natural abundance, low cost, and superior chemical stability in aqueous
solution [
35
,
45
,
59
,
82
,
89
,
105
]. However, hematite has a few severe drawbacks
that limits its application as photoelectrode. First, the conduction band of hematite
is more positive than the water reduction potential, as a result, a large external bias
is needed to drive water splitting. Furthermore, hematite has a very short excited
state lifetime (*10 ps) and a short hole diffusion distance (2-4 nm), which cause
significant loss via electron-hole recombination [
47
,
48
,
100
]. In this regard, very
thin layer of hematite is required to reduce the diffusion length for minority carrier
and facilitate charge separation. Nanostructured materials open up new opportu-
nity to address this issue by providing extremely large surface area, as well as short
hole diffusion distance. For instance, Lin et al. reported the fabrication of ultrathin
hematite thin films on TiSi
2
nanonet using atomic layer deposition technique
(Fig.
11
)[
46
]. This core shell structure has several advantages over bulk structure.
The TiSi
2
scaffold provides large surface area and the TiSi
2
is also highly con-
ductive, which is beneficial for charge transport. Besides, the ultrathin hematite
Search WWH ::

Custom Search