Environmental Engineering Reference
In-Depth Information
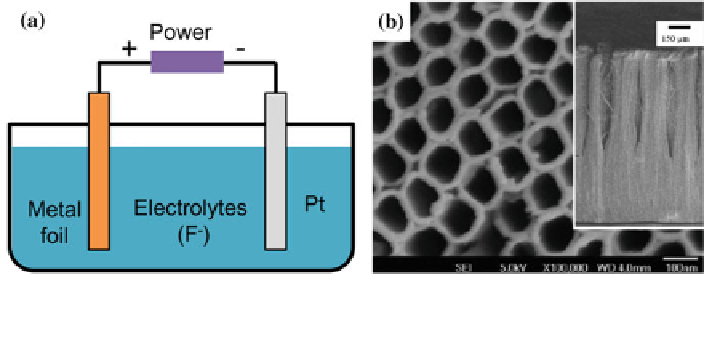
Fig. 5 a A schematic diagram of a two-electrode electrochemical cell for anodization. b SEM
images of TiO
2
NTAs prepared by potentiostatic anodization of Ti foils in an ethylene glycol
electrolyte containing NH
4
F and H
2
O. Reproduced with permission from [
70
]
obtained by anodic oxidization in aqueous or organic electrolytes containing
fluoride ions. For example, Cai et al. investigated the effect of electrolyte com-
position on the fabrication of TiO
2
NTAs. By adjusting the pH of electrolyte
using different additives, TiO
2
NTAs with different lengths ranging from 300 nm
to 6.4 lm were formed in an aqueous electrolyte containing potassium fluoride
[
4
]. Schmuki et al. synthesized TiO
2
NTAs with a length of up to 4 lmina
neutral fluoride solution containing phosphate [
20
]. Due to the high chemical
dissolution rate in an aqueous solution containing fluoride ions, the length of the
prepared TiO
2
NTAs was short. In comparison to aqueous electrolytes, much
longer TiO
2
NTAs can be formed in polar organic electrolytes due to low
chemical dissolution rate resulting from low water content. The most commonly
used organic electrolyte is ethylene glycol [
77
], glycol [
57
], acetic acid [
91
],
formamide (FA) [
81
], and dimethylsulfoxide (DMSO) [
116
]. By using organic
electrolyte, the length of TiO
2
NTAs could be extended to up to 100 lm under
the proper anodization conditions in organic electrolyte [
70
,
71
]. Grimes and co-
workers have widely explored the organic electrolytes in preparing the TiO
2
NTAs and have made significant progress. Recently, they prepared highly
ordered TiO
2
NTAs of over 1,000 lm in length and aspect ratio about 10,000 by
potentiostatic anodization of Ti foils in an ethylene glycol electrolyte containing
NH
4
F and H
2
O[
70
]. The length and the wall thickness of the TiO
2
NTAs were
readily controlled by adjusting the electrochemical parameters such as the
anodization duration, the composition and temperature of the electrolyte, and the
anodization voltage. Besides TO
2
nanotube, other metal oxide nanotube and
nanoporous nanostructures have developed such as Fe
2
O
3
[
39
,
109
], WO
3
[
118
,
120
], Nb
2
O
5
[
19
], and Ta
2
O
5
[
15
,
40
] via the same electrochemical anodization
method. These research works have demonstrated electrochemical anodization to
be
an
effective
approach
to
fabricate
high
surface
area
metal
oxide
nanostructures.
Search WWH ::

Custom Search