Environmental Engineering Reference
In-Depth Information
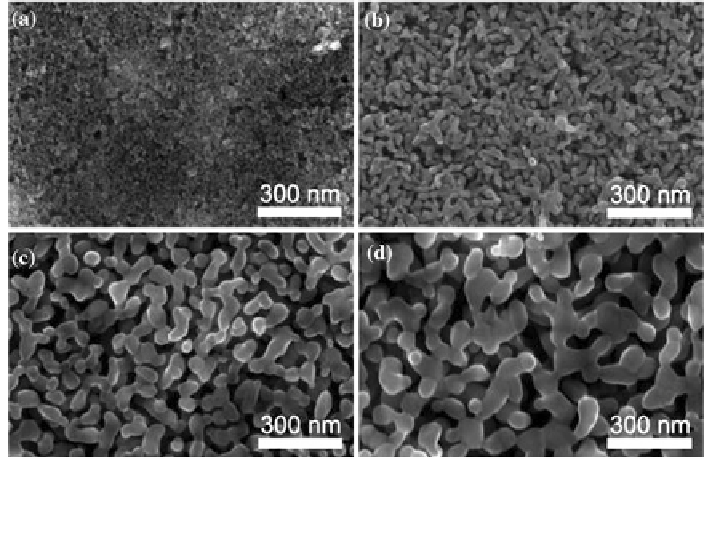
Fig. 4 2 Scanning electron micrographs of mesoporous hematite films prepared on SiO
2
/F:SnO
2
substrates after different heat treatments: a As deposited with porogen b After 10 h at 400 C,
and c After 20 min at 700 C and d 800 C. Reproduced with permission from [
83
]
from aqueous solutions at room temperature [
58
]. The multifaced 3D structure act
as a light-scattering layer, which can significantly improves the photocurrent by
reducing the loss via surface light reflection.
2.4 Electrochemical Anodization Method
Electrochemical anodization of metal foils is a simple and convenient method for
creating ordered and self-oriented porous metal oxide nanostructures perpendic-
ular to the substrate with controllable pore size and length, especially for nanotube
arrays (NTAs) [
21
,
60
,
70
,
71
,
104
]. Anodization of metal foils is usually con-
ducted in a conventional two-electrode electrochemical cell with a platinum foil as
cathode at a constant potential. A schematic diagram for the anodization of metal
foils is illustrated in Fig.
5
.
A type example is electrochemical anodization of Ti foil to prepare vertically
and highly ordered TiO
2
NTAs. In 2001, Grimes and co-workers first reported the
formation of TiO
2
NTAs via electrochemical anodization of Ti foil in a hydro-
fluoric electrolyte [
21
]. Further studies focused on the precise control the mor-
phology of TiO
2
NTAs such as pore size, length, and wall thickness. Electrolyte
composition plays a critical role in determining the morphology and structure of
TiO
2
NTAs. So far, TiO
2
NTAs with various diameter and length are easily
Search WWH ::

Custom Search