Environmental Engineering Reference
In-Depth Information
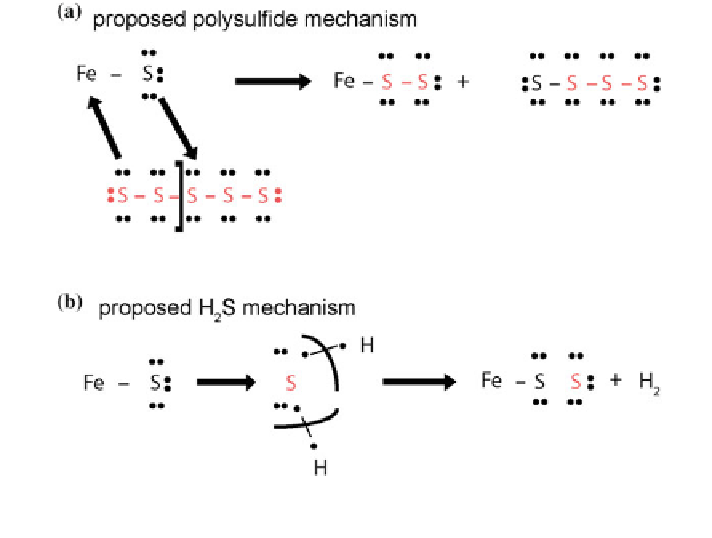
Fig. 2 a Proposed mechanisms of pyrite formation through a FeS intermediate. Reprinted with
permission from [
36
]
2.2 Hydrothermal Synthesis of Nano Pyrite
Hydrothermal methods have been used extensively in nanocrystal synthesis in the
past [
3
,
25
,
43
]. This method makes use of a stainless steel digestion bomb that is
typically Teflon lined. The buildup of pressure in the container allows for lower
temperature to be used instead of high temperature ambient pressure synthesis; care
must be used during use due to this pressure buildup, which is why these systems
have earned the nickname ''bomb.'' The use of a single precursor is usually used,
and through the decomposition of the molecule produces the reactive species.
Greater control of the system is achieved due to lack of variables such as injection
rate and injection temperature. Having a single precursor though limits you to only
a few precursors and are expensive. Another drawback of these systems is the time
required to carry out the reaction (*24 h) and the lack of the ability to take timed
aliquots of the sample during synthesis to study reaction progression. Even with
these drawbacks, it is still used for its simplicity and control.
One of the first reports of utilizing hydrothermal synthesis methods come from
Chen et al. [
43
]. In this chapter, an iron Diethyldithiocarbamate (Fe(S
2
CNEt
2
)
3
)
complex was utilized for the single precursor, and upon completing the reaction
cubic FeS
2
crystallites with *500 nm edge lengths were obtained. It was shown
that a minimum temperature of 180 C was necessary to achieve pure phase pyrite,
and that lower temperatures produced marcasite impurities. These crystallites
Search WWH ::

Custom Search