Environmental Engineering Reference
In-Depth Information
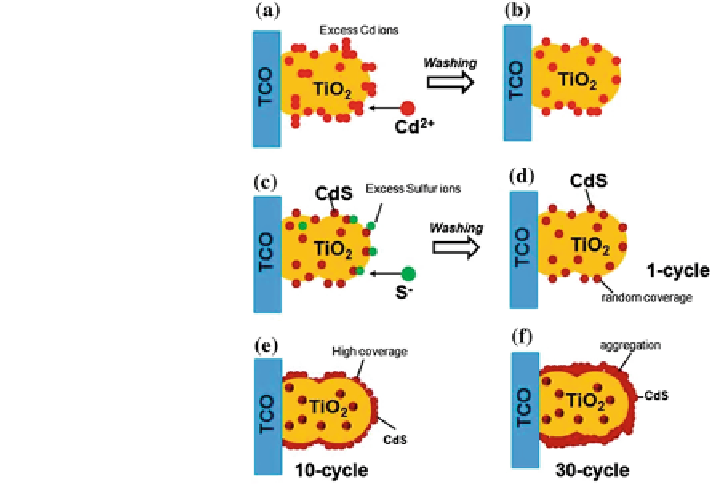
Fig. 3 Schematic illustration
of SILAR process
(a) Adsorption of cationic
ions (Cd
2+
)(b) rinsing (I)
removes excess,
nonspecifically adsorbed
Cd
2+
(c) reaction of anionic
(S
-
) with preadsorbed Cd
2+
ions to form CdS and
(d) rinsing (II) to remove
excess and unreacted species
and form the solid solution
CdS on surface of the
substrate. The coverage of
CdS at higher cycles (e)10
and (f)30
redissolved into chemical bath and QDs films might feel-off from the coating
surface. During first SILAR cycle, the seed layer of QDs was formed on the
coating surface and directing further growth for successive coating cycles. The
influence of coating cycles on growth of QDs can be studied by optical absorption
spectra. Figure
4
a-c explains the influence of coating cycles on optical absorption
of CdS, CdSe [
77
], and Sb
2
S
3
[
78
], respectively. From Fig.
4
a, it is clearly
understood that the absorption of CdS and CdSe is found to increase by gradually
improving the coating cycles. Interestingly, nucleation and growth of CdSe on
TiO
2
can be greatly accelerated with a CdS underlayer, where CdS is rather a
promoter for the preferential growth of CdSe (Fig.
4
b) as it has been also observed
for CBD.
Typically, semiconductor QDs by SILAR process has been demonstrated under
aqueous medium, but the high surface tension causes poor wetting ability on a
solid surface, which leads to poor penetration of the solution in a porous matrix.
Therefore, low surface tension solvent is recommended like alcohol solutions for
efficient QDs coating. Since it has high wettability and superior penetration ability
on the mesoscopic TiO
2
film, well-covered QDs on the surface of mesopores is
achieved easily. The high coverage of QDs results by alcohol solvent showed high
absorbance than that of aqueous solvent (Fig.
4
c).
Search WWH ::

Custom Search