Environmental Engineering Reference
In-Depth Information
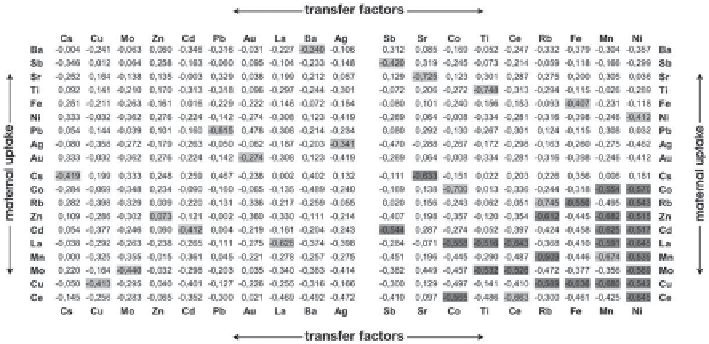
Fig. 6.3
Analyzes of correlation between element-specific transfer factors ((d/kg), vertical col-
umns) vs. element-specific maternal uptake amounts (µg/d) via food to determine synergistic or
antagonistic effects.
Dark grey
shadowed values: significant correlations (r ≥ 0.5),
light-grey
: self-
correlation effects (Wünschmann
2007
; Wuenschmann et al.
2008
)
A more general problem is why TF value and partition for, for example, Zn are
that much higher than for those for Mn. Possible reasons include interactions of the
elements with one another but also effects on the transport of other elements, e.g., if
they compete for carrier binding sites. Given the latter, TF values should be a com-
plex function of different chemical factors. The only practical way to address this
problem is to correlate (nonaveraged, individual) TF values of one element with the
amounts consumed of another (or this very) element consumed by the mother. If so,
the TF of one element significantly may responds to changing uptake amounts of
another one, which causes an influence—either synergistic or antagonistic—which
could be detected in TF variations. Correlation diagrams for the corresponding data
are summarized in Fig.
6.3
. There are no significant influences of maternal intake
amounts of other elements to TF values of Pb or Cd.
The relatively low TF of Cd (0.014 d/kg) is interpreted as a result of its con-
siderably lower stability of complexes as compared to those of for example Ni,
Zn or Cu (0.054, 0.077 and 0.156 d/kg) (cf. data in (Irving and Williams
1953
))
therefore biochemical transport of Cd and some other metals during production
of milk will take place mainly as simple aquaions, much like with alkali metals
and alkaline earths (Neville
1991
; Wuenschmann et al.
2004b
). Accordingly, Cd
(or Sr or Ba) cannot effectively compete with other metals—neither such supplied
in far larger amounts like Fe, Zn, Mn or Cu nor those which are similarly rare
(10-20 µg/d ≈ 100-200 nmol/d like Y, Cs, Co or Zr)—for carriers. The latter carrier
may be both transport proteins and ligands, the amounts of which in milk for more
than outweigh the metal contents (chloride, citrate, oxalate, some amino acids like
glutamate (Wuenschmann et al.
2004a
) Thus, there is no effect of other elements on
the TF of Cd and its partition into milk. Although there is no corresponding effects
for Pb in these data, the fragmentary data on complex stability between Pb
2+
and
“milk ligands” preclude similar statements like those referring to Cd.