Agriculture Reference
In-Depth Information
Figure 7.2
Biogeochemistry of
carbon equilibrium. The
processes that release
carbonates are responsible for
much of the buffering capacity of
natural soils against the effects of
acid rain.
Precipitation
CO
2
Microbial
Degradation
CO
2
Topsoil (A Horizon)
Subsoil
Horizons (B)
CO
2
+
H
2
O
2
CO
3
Carbonic acid
Limestone and
Dolomite Parent Rock
Ca(HCO
3
)
2
CaCO
3
(s)
+
H
2
CO
3
MgCO
3
(s)
+
H
2
CO
3
Mg(HCO
3
)
2
buffering capacity of natural soils against the effects of acid rain. Thus, carbonate-
rich soils such as those of central North America are able to withstand even
elevated acid deposition compared to thin soil areas such as those in the Canadian
Shield, the New York Finger Lakes region, and much of Scandinavia.
The concentration of carbon dioxide is constant, since the CO
2
in solution is
in equilibrium with the air, which has a constant partial pressure of CO
2
. The
two reactions and ionization constants for carbonic acid are
HCO
3
−
+
H
3
O
+
10
−
7
H
2
CO
3
+
H
2
O
↔
K
a
1
=
4
.
3
×
(7.6)
HCO
3
−
+
CO
3
2
−
+
H
3
O
+
10
−
11
H
2
O
↔
K
a
2
=
4
.
7
×
(7.7)
K
a
1
is four orders of magnitude greater than
K
a
2
, so the second reaction can
be ignored for environmental acid rain considerations. The solubility of gases
in liquids can be described quantitatively by Henry's law, so for CO
2
in the
atmosphere at 25
◦
C, we can apply the Henry's law constant and the partial
pressure to find the equilibrium. The
K
H
value for CO
2
10
−
2
mol
L
−
1
atm
−
1
. We can find the partial pressure of CO
2
by calculating the fraction
of CO
2
in the atmosphere. Since the mean concentration of CO
2
in Earth's
troposphere is 350 ppm by volume in the atmosphere, the fraction of CO
2
must
be 350 divided by 1 million, or 0.000350 atm.
Thus, the carbon dioxide and carbonic acid molar concentration can now be
found:
=
3.4
×
10
−
5
M
10
−
2
mol L
−
1
atm
−
1
[CO
2
]
=
[H
2
CO
3
]
=
1
.
2
×
=
3
.
4
×
×
0
.
000350 atm

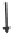














Search WWH ::

Custom Search