Environmental Engineering Reference
In-Depth Information
Inward diffusion
O
2
and N
2
Heterocyst
Vegetative cells
Vegetative cells
PSI
ADP > ATP
Glutamic acid
Glutamic acid
Glutamic acid
Glutamine
Nitrogenase, n-fixation
N
2
+ATP + NADPH > NH
4
+
GOGAT
Oxygenic photosynthesis
(PSII + PSI) production of sugars
Glutamine
-oxoglutaric
acid
α
Respiratory processes utilizing
O
2
and producing NADPH
Layered wall impermeable
to diffusion of gasses
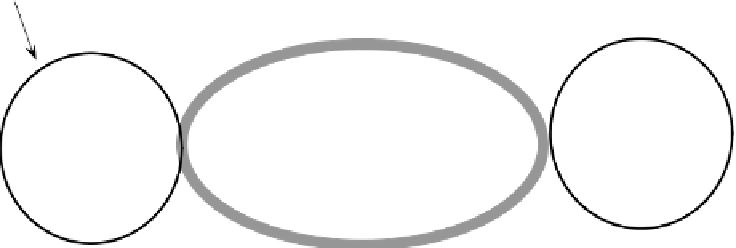
FIGURE 13.3
A diagram of cyanobacterial vegetative cells, a heterocyst, adaptations to pro-
tect nitrogenase from deactivation by O
2
, and mode of N transport from the heterocyst into
vegetative cells.
form aggregations in which O
2
is depleted. Heterocysts and patterns of ag-
gregation allow low O
2
concentrations while promoting high-energy avail-
ability through respiration for the costly process of nitrogen fixation (Paerl
and Pinckney, 1996). The molecular control over nitrogen fixation has
been documented (Böhme, 1998), and the chemical signaling controlling
heterocyst formation has been described (Yoon and Golden, 1998). Chem-
ical signaling controlling heterocyst formation is particularly interesting
because it is the first documented case of intercellular signaling with a
peptide in the Bacteria.
Some wetland and riparian species of plants are associated with nitro-
gen-fixing bacteria. Alder trees have symbiotic bacteria that fix N
2
. The
fixed nitrogen may enter aquatic habitats through leaching or movement
of leaves into the aquatic habitat. Some wetlands can also have significant
populations of nitrogen-fixing cyanobacteria. For example, the aquatic fern
Azolla
has endosymbiotic, nitrogen-fixing cyanobacteria.
Azolla
growth is
encouraged in traditional rice culture. The field is drained to release the
fixed nitrogen and then flooded and planted with rice. Cyanobacterial
crusts associated with sediments in wetlands can also fix significant amounts
of nitrogen.
Nitrogen can also be fixed in the atmosphere when lightning produces
enough energy to cause N
2
and O
2
to combine and form nitrate. Thus,
rainwater naturally contains nitrate. Additional nitrogen is found in rain-
fall and particulates in the atmosphere that are suspended from terrestrial
systems. Burning of fossil fuels introduces nitrogen oxides into the atmos-
phere. In aquatic systems with low amounts of nitrogen, atmospheric de-
position can be a significant source of nitrogen. Nitrogen is also fixed from
the atmosphere during the industrial production of fertilizers. This process
has approximately doubled worldwide rates of nitrogen fixation and led to











Search WWH ::

Custom Search