Environmental Engineering Reference
In-Depth Information
Oxic processes
Oxygenic photosynthesis
Respiration
Heterotrophy
Methanotrophy
Organic
carbon
Methane
Inorganic carbon
Methanogenesis
Fermentation
and anoxic respiration
Anoxygenic photosynthesis
Anoxic processes
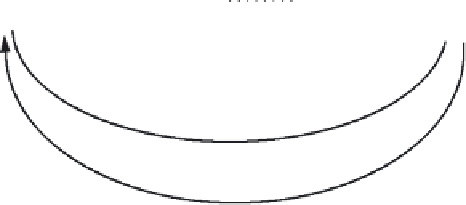
FIGURE 12.6
A diagram of the generalized carbon cycle.
Each of the nutrient cycles has its own idiosyncrasies related to the differ-
ent chemical properties of the individual nutrient and the conventions of
researchers.
The carbon cycle is diagrammed using this technique (Fig. 12.6). CH
4
is listed separately from other organic forms because of its crucial role in
global carbon cycling. Also, photosynthesis is an assimilation flux, but the
processes of oxic and anoxic photosynthesis are different, so they are sep-
arated in this chart.
SUMMARY
1. Inorganic carbon is found in the form of carbon dioxide (CO
2
) gas in
the atmosphere at about 350 ppm. This gas readily dissolves in water,
in which it can take the form of CO
2
, carbonic acid (H
2
CO
3
),
bicarbonate (HCO
3
), and carbonate (CO
3
).
2. The equilibration between the different ionic forms of inorganic
carbon is called the bicarbonate equilibrium. One of the most
important factors driving the equilibrium is the pH of the water.
Under low pH, CO
2
is formed; under high pH, the equilibrium moves
toward carbonate. The equilibration can buffer natural waters against
changes in pH.
3. Organic carbon can be dissolved or particulate; the particulate
fractions traditionally are divided into fine and coarse components.
Dissolved organic material can be divided into humic and nonhumic
substances. The nonhumic substances generally have low molecular
weight and are metabolized easily by aquatic microbes. The humic
substances have high molecular weight and are more resistant to
breakdown.
4. Organic carbon is produced by oxygenic (O
2
-producing) and
anoxygenic photosynthesis. Organic carbon is oxidized to CO
2
by a
















Search WWH ::

Custom Search