Environmental Engineering Reference
In-Depth Information
The amount of O
2
dissolved in water is a function of many factors, in-
cluding metabolic activity rates, diffusion, temperature, and proximity to the
atmosphere. This amount can be expressed in several related concentration
units, including mg liter
1
, mol liter
1
, and percentage saturation. The per-
centage saturation is the concentration of O
2
relative to the maximum equi-
librium concentration for that solution.
Dissolved oxygen
refers to the O
2
dissolved in water (as opposed to oxygen that is part of other chemical
compounds).
The
saturation concentration
of O
2
is determined as the equilibrium
concentration when pure water is in contact with the atmosphere for an
extended period of time. The amount of O
2
that can be dissolved in water
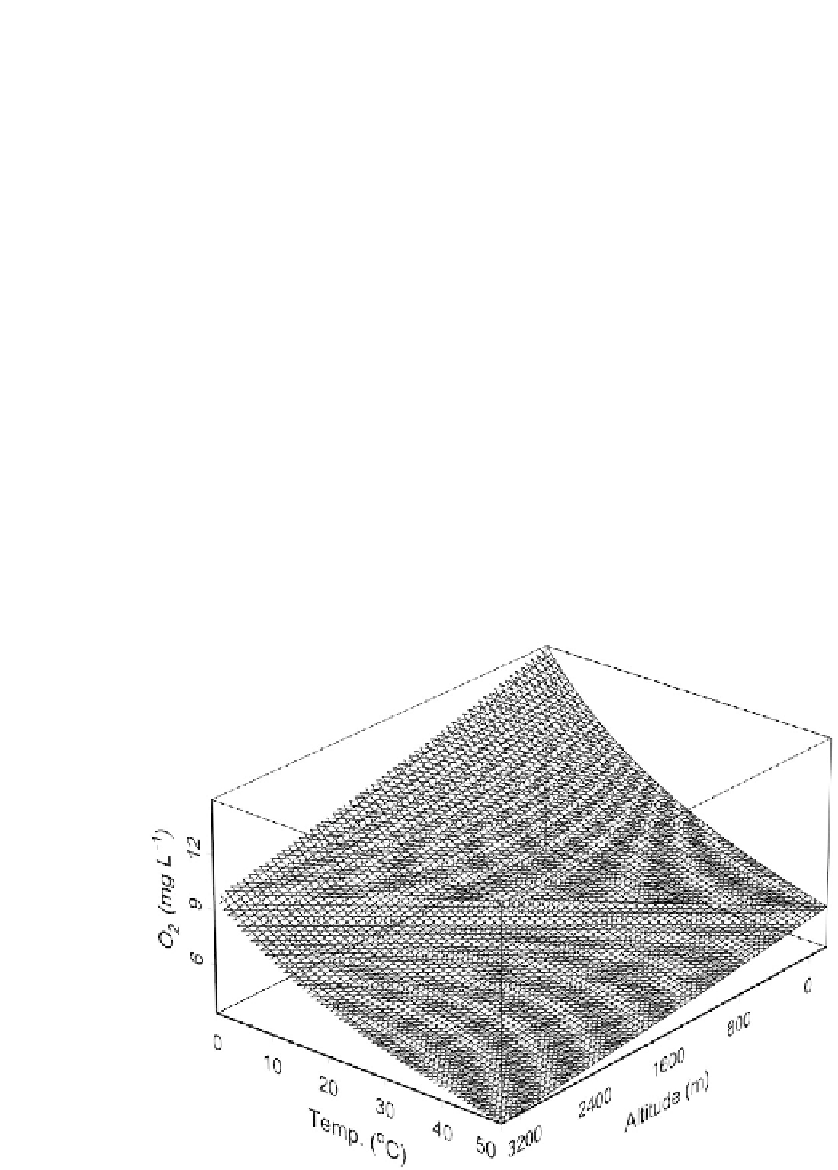
is a function of temperature; the lower the temperature, the greater the
concentration of O
2
under equilibrium conditions (Fig. 11.8). In addition,
the greater the atmospheric pressure, the greater the saturation O
2
con-
centration. Atmospheric pressure is a function mainly of altitude (Fig.
11.8), but there is also variation with barometric pressure (not shown). Fi-
nally, O
2
concentration increases with increasing water pressure (i.e., depth
in the water) and decreases with increasing salinity.
A point of confusion for some students is the concept that O
2
con-
centrations can exceed the level of saturation. If O
2
becomes highly super-
saturated, it will form bubbles and come out of solution. However, at con-
centrations severalfold greater than saturation, O
2
can remain dissolved
and slowly equilibrate with the atmosphere.
FIGURE 11.8
Saturation concentrations of dissolved O
2
as a function of temperature and
altitude. The equation that describes the curve is Ln (O
2
)
10
4
2.692
1.27
(alt)
10
10
(alt)
2
10
4
(temp)
2
10
6
(temp)
3
, where
6.15
0.0286 (temp)
2.72
2.09
mg liter
1
, alt is altitude in meters, and temp is temperature in °C (equations modified
from Eaton
et al.,
1995).
O
2
Search WWH ::

Custom Search