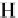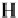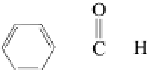Environmental Engineering Reference
In-Depth Information
H
-C=O
|
in their chemical structure. The carbonyl is in a terminal position in alde-
hydes. A compound is described as an aldehyde if it has one terminal car-
bonyl, a dialdehyde if it has two, and a trialdehyde if it has three carbonyls.
Aldehydes include saturated (single bonds) aliphatic, unsaturated (one
or more double bonds) aliphatic, and aromatic or cyclic compounds. Satu-
rated aliphatic aldehydes include formaldehyde (one carbon), acetalalde-
hyde (two carbons), propionaldehyde (three carbons), butryaldehyde (four
glutaraldehyde is a dialdehyde with carbonyls on both ends of the molecule.
Unsaturated aliphatic aldehydes contain a carbon-carbon double bond
tonaldehyde. Methacrolein is commonly used to produce methyl methacry-
late, an eye-irritating ester used as an adhesive in many industrial applica-
tions. Aromatic aldehydes include compounds such as benzaldehyde and
cinnamaldehyde.
Table 4.1
Chemical Structures and Properties of Common Aldehydes
Solubility
(g/L)
Compound
Structure
M. W.
O
Formaldehyde
30.03
560
H
C
H
O
Acetaldehyde
44.05
200
CH
3
C
H
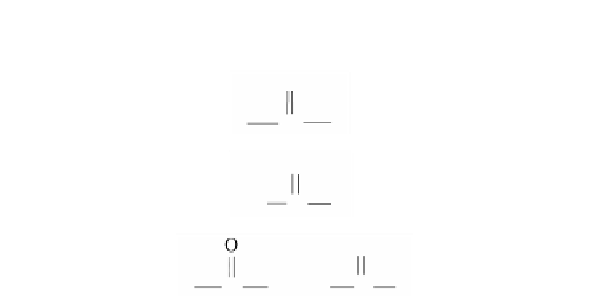
Glutaraldehyde
100.12
Miscible
O
Acrolein
56.06
210
CH
2
CH
C
H
O
Crotonaldehyde
76.09
181
H
CH
3
CH
CH
C
Benzaldehyde
106.11
3
Source:
From Leikauf, G.D., in
Environmental Toxicants: Human Exposures and
their Health Effects
Van Nostrand Reinhold (John Wiley &
Sons), New York, 1992, chap. 2. With permission.
, Lippman, M., Ed.
,