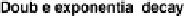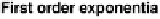Environmental Engineering Reference
In-Depth Information
ER= ER
0
e
-kt
(9.5)
where ER
0
= initial emission rate, mg/m
2
(hr)
e
= natural log base
k
= first order decay constant (hr
-1
)
t
= time (hr)
When early emission rates are much faster than long-term emission rates,
they may be better described by a double exponential model:
ER = ER
01
e
-k1t
+ ER
02
e
-k2t
(9.6)
where ER
01
= the initial emission rate associated with evaporation, or in the
case of UF-bonded wood products, free HCHO and HCHO
released by hydrolysis
ER
02
= the emission rate associated with diffusion, or in the case of
HCHO, UF resin hydrolysis
k
1
= decay rate for initially rapid emissions (hr
-1
, day
-1
)
k
2
= decay rate for longer term emissions (hr
-1
, day
-1
)
t
= time (hr, day)
Decay constants (k
1
, k
2
) in the double exponential model must be empirically
derived.
As can be seen in
Figure 9.12
,
the first-order exponential decay model
predicts actual decreases in emission rates for HCHO emissions from particle
board in a large dynamic chamber very well for the first couple of weeks,
but poorly thereafter; the double exponential model, on the other hand,
provides a much better fit for measured HCHO emission/concentration data
over a 6-month period.
Figure 9.12
Emission decay rates of formaldehyde from particle board predicted by
first order decay and double-exponential models. (From Brown, S.K.,
Indoor Air
, 9,
209, 1999. With permission.)