Environmental Engineering Reference
In-Depth Information
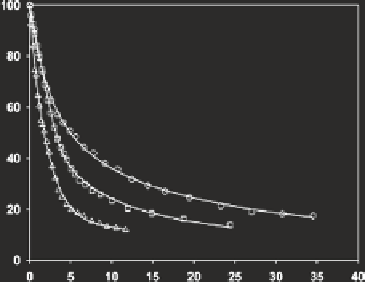
Fig. 4
Multisite Stern-
Volmer (MSV)-fitted
fluorescence quenching data
of soil fulvic acid (15 mg
L
−
1
) titrated with Cu
2
+
at
0.1 M ionic strength: (
circles
)
pH 5 data; (
squares
) pH 6
data; (
triangles
) pH 7 data;
(
solid
lines
) MSV predicted
intensity values.
Data source
Ryan and Weber (
1982a
)
Added Cu
2+
[M × 10
5
] (C
M
)
3.3 Theory for Conditional Stability Constant of M-DOM
Complexation
The conditional stability constant of a M-DOM complex is operationally defined
as the binding efficiency of the newly formed bond between the functional group
of the DOM component (acting as an organic ligand) and the trace metal ion M,
when they are mixed up under specific conditions in aqueous media. Conditional
stability constants of a M-DOM complex can be useful to characterize the formed
complex, to apply the strong binding capacity of organic substances to control spe-
ciation, toxicity, bioavailability and fate of toxic metals used e.g. in industries, and
for predicting biological effects of metals in natural water, sediment and soil envi-
ronments (Shcherbina et al.
2007
; Mostofa et al.
2011
; Sekaly et al.
2003
; Huber
et al.
2002
; Filella et al.
2007
; Hörnström et al.
1984
; Hughes et al.
1995
; Markich
2002
; Matsumoto et al.
2005
; Martel and Motekaitis
1988
).
A conditional stability constant has been determined by Midorikawa and
Tanoue (Appendix A) (Midorikawa and Tanoue
1998
), adopting the relationship
between measured fluorescence intensity and complexation for a divalent metal
ion (M, ca. Cu
2
+
) with organic ligands, and assuming a 1:1 stoichiometry (Ryan
and Weber
1982a
). The complexing reactions that fit the experimental data can be
described by the linear regression program. The relationship between measured
fluorescence intensity and complexation can be described as follows (Eq.
3.1
)
(Ryan and Weber
1982a
):
[
ML
]
C
L
F
0
−
F
F
0
−
F
end
X
=
=
(3.1)
where the quantity [ML]/
C
is the fraction of the ligand bound to the metal to form
the complex ML. Such a fraction can be expressed in terms of the measured fluo-
rescence intensity,
F
.
F
0
and
F
end
are the limiting intensities before and after metal
titration. They correspond to the intensities when all ligands are entirely free and
bound, respectively.