Environmental Engineering Reference
In-Depth Information
fluorescent DOM (or functional groups or chromophores) are likely responsible for the
formation of complexes with trace elements.
The key fluorophores in allochthonous fulvic and humic acids, tryptophan
amino acid and protein in natural waters are composed of Schiff-base derivatives
(-N
=
C-C
=
C-N-), -COOH, -COOCH
3
, -OH, -OCH
3
, -CH
=
O, -C
=
O, -
NH
2
, -NH-, -SH, -CH
=
CH-COOH, -OCH
3
, -CH
2
-(NH
2
)CH-COOH, S-, O- or
N-containing aromatic compounds, and so on (Malcolm
1985
; Mostofa et al.
2009a
;
Mostofa and Sakugawa
2009
; Senesi
1990
; Steelink
2002
; Leenheer and Croue
2003
; Fu et al.
2007
; Corin et al.
1996
; Peña-Méndez et al.
2005
; Seitzinger et al.
2005
; Zhang et al.
2005
). The complexation of trace elements with fluorescent sub-
stances also affects the fluorescence peak position of the respective fluorophore.
Usually, both excitation and emission wavelengths of the respective peak position
gradually shift toward the longer wavelength with increase in the reaction time (Wu
et al.
2004a
,
c
; Plaza et al.
2006
). It has also been found that comparison of the EEM
spectra before and after binding in metal-DOM complexes shows that the fast bind-
ing site in fulvic acid is responsible for 71-87 % of the total fluorescence decrease,
while the remainder is associated with the slow binding site (Wu et al.
2004c
).
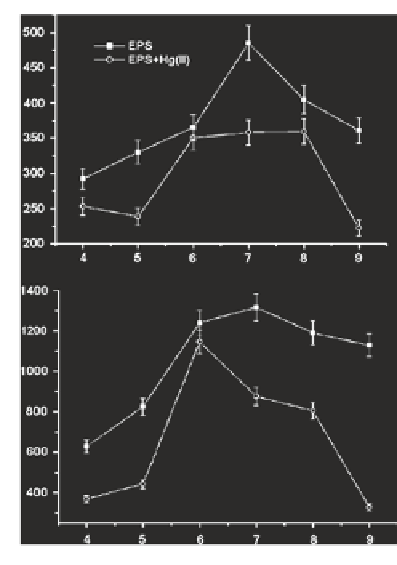
EPSs show two fluorescence peaks (peak T and T
UV
) in the absence and pres-
ence of trace elements such as Hg
2
+
, with the maximal fluorescence intensity at
neutral pH (Fig.
2
) (Zhang et al.
2010
). The excitation-emission matrix (EEM)
Fig. 2
Changes in the
fluorescence intensities
of peak T (
a
) and peak
T
UV
(
b
) for extracellular
polymeric substances
(EPS) with solution pH in
the absence and presence
of 3.0 mg L
(a)
−
1
Hg(II).
The error bar indicates the
standard deviation of three
independent measurements.
Data source
Zhang et al.
(
2010
)
Peak T
(b)
Peak T
UV
pH