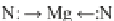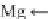Environmental Engineering Reference
In-Depth Information
(a)
(b)
Fig. 7
The possible resonance configuration of Mg with
π
-electrons of two N-atoms located in
the chlorophyll
a
structure (
a
) and chlorophyll
a
dimer (
b
). Only the two N-atoms in porphyrin
ring with Mg are presented in the structure to simplify the resonance structure
primary electron release in PSII involves the chlorophyll
a
dimer (Boussaad et al.
1997
; Nilsson Lill
2011
). This can be justified by the theory of excitation of mul-
tiple functional groups bound to macromolecular organic substances (e.g. fulvic
acids or humic acids). Light excitation is expected to induce first the release of the
electron less strongly bound in the relevant functional groups, and then of the sub-
sequent ones (see chapter
“
Colored and Chromophoric Dissolved Organic Matter
It is hypothesized that the first electron is released from the
π
-bonding sys-
tem formed between two N-atoms in the porphyrin ring and Mg. In fact, Mg
(1
s
2
2
s
2
2
p
6
3
s
1
3
p
x
1
3
p
y
0
3
p
z
0
) can form two covalent bonds with two N-atoms of
the porphyrin ring using 3
s
1
and 3
p
x
1
orbitals, whilst other two empty 3
p
y
0
and
3
p
z
0
orbitals can accept the
π
-electrons from the remaining two N-atoms. The
π
-bonding systems among these orbitals (3
p
y
and 3
p
z
) can interchange with one
another because of the similar energy levels. Therefore, one can have resonance
configuration upon exchange of electrons between the orbitals and Mg (Fig.
7
a).
Chl
a
dimer is formed through hydrogen bonding via H
2
O bridges, and H
2
O is the
key component in the formation of such dimers (Shipman et al.
1976
; Hynninen
and Lötjönen
1993
; Boussaad et al.
1997
; Catalan et al.
2004
). It is supposed that
hydrogen (H)-bonding is formed between the non-bonding
π
-electrons of two
N-atoms in the porphyrin ring. The latter is also a resonance structure where elec-
trons can move through the whole Chl
a
dimer (Fig.
7
b).
The formation of H-bonds through H
2
O bridges is suggested by earlier studies
(Shipman et al.
1976
), and can be justified by the shift of the
π
-bonding system
in H-N-Mg-N-H (Fig.
7
b). This system can assist the release of electrons in a
much easier way than the single N-Mg-N system (Fig.
7
a). Based on multimer
model studies one obtains equal site energies and inhomogeneous widths for all
pigments, which leads to similar distances and to nearest-neighbor dipole-dipole
interactions between the central chlorin cofactors (Durrant et al.
1995
; Renger and
Marcus
2002
; Barter et al.
2003
). This may result into two wavelength positions
for the electronic states in the reaction center (RC): uncoupled Chls can absorb at
670 nm, and electronically coupled chlorins (the central cofactors) or Chl dimers