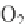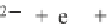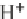Environmental Engineering Reference
In-Depth Information
In the reactions above, release of O
2
occurs not from H
2
O but from H
2
O
2
.
Correspondingly, photosynthetic O
2
evolution would involve differ-
ent stages that carry out a gradual accumulation of oxidizing equivalents in the
Mn-containing water-oxidizing complex (WOC) (Samuilov et al.
2001
). The
WOC can exist in different oxidation states (S
n
, where high n indicates the most
oxidised states), which can be probed by addition of different redox-active mol-
ecules. The interaction of H
2
O
2
with the S states of the WOC is depicted in the
scheme that follows (Velthuys and Kok
1978
; Mano et al.
1987
; Samuilov et al.
2001
; Latimer
1952
; Ilan et al.
1976
; Samuilov
1997
):
E
0
= 1.77 V
H
2
O
2
+ 2H
+
2H
2
O
S
-1
S
0
S
2
S
1
O
2
•
-
+ 2H
+
H
2
O
2
O
2
+ 2H
+
H
2
O
2
+ 2H
+
E
0
= 1.71 V
E
0
= 0.69 V
These studies suggest that H
2
O
2
is an evolutionary precursor of H
2
O as
the electron donor for PSII in cyanobacteria (Bader
1994
; Samuilov
1997
;
Blankenship and Hartman
1998
).
The release of O
2
from H
2
O
2
instead of H
2
O can be justified by the rapid for-
mation of H
2
O
2
and of highly reactive chemical forms collectively denoted as
'reactive oxygen species (ROS)'. Both H
2
O
2
and ROS are formed from O
2
when
it is exposed to high-energy or electron-transfer chemical reactions, which can be
expressed as follows (Chance et al.
1979
; Koppenol
1976
; Klotz
2002
; Apel and
Hirt
2004
):
3O
2
+
h
υ →
1
O
2
→
1
O
2
(3.16)
H
+
3O
2
+
e
−
+
h
υ →
O
2
•−
−→
HO
2
•
(3.17)
2H
+
−→
H
2
O
2
O
2
•−
+
e
−
+
h
υ →
O
2
2
−
(3.18)
(3.19)
2H
+
−→
H
2
O
O
−
+
e
−
+
h
υ →
O
2
−
(3.20)