Environmental Engineering Reference
In-Depth Information
that different types of plant material lead to different rates of acetate formation.
There is also a stronger substrate-based coupling of root surface and methanogens
in oligotrophic (bog) than in minerotrophic (fen) sites (Cadillo-Quiroz et al.
2010
;
Ström et al.
2003
; Öquist and Svensson
2002
). Seasonal algal or phytoplankton
blooms might be responsible for formation of acetate and CH
4
in the sediments
of deep lakes (Schulz and Conrad
1995
). The acetate concentration profiles show
maxima (~100
μ
M in 2 or 4 cm depth) in summer and minima (~5
μ
M over the
entire depth) in winter, when the respective CH
4
concentrations are ~750
μ
M in
summer and ~120
μ
M in winter (Schulz and Conrad
1995
).
It is evidenced that gas bubbles contain about 60-70 % CH
4
with an average
δ
13
C of -56.2 % and
δ
D of -354 %, and 2 % CO
2
with an average
δ
13
C of -14.1 %
(Thebrath et al.
1993
). These data indicate that CH
4
is produced from methyl car-
bon,
i.e.
mainly using acetate as fermentative substrate (Thebrath et al.
1993
).
In anoxic paddy soil, interspecies H
2
transfer within methanogenic bacterial asso-
ciations (MBA) account for 95-97 % of the conversion of
14
CO
2
to
14
CH
4
, and
only 3-5 % of the
14
CH
4
is produced from the turnover of dissolved H
2
(Conrad
et al.
1989a
,
b
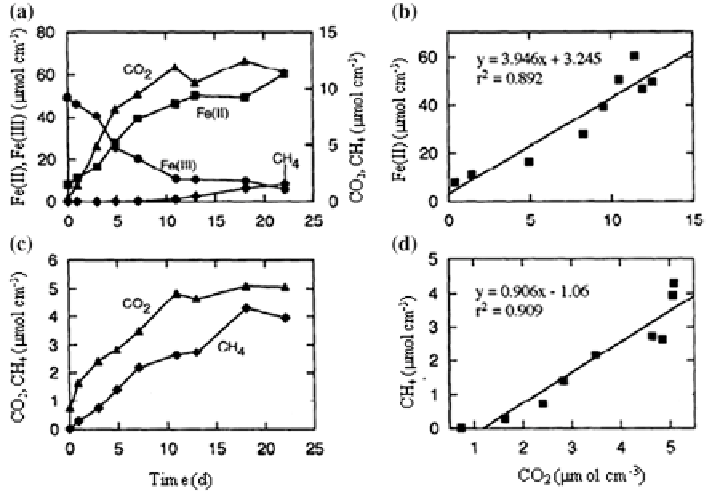
). An experimental study demonstrates that the ratio of Fe(II) pro-
duction to CO
2
production (3.9) is similar to that expected (4.0) for organic carbon
oxidation coupled to Fe(III) oxide reduction (Fig.
8
) (Roden and Wetzel
1996
).
The study also shows that the rates of CH
4
production are low during the Fe(III)
reduction in oxidized sediments, but increase when the Fe(III) oxides are depleted
to background levels (Fig.
8
a). The rates of CO
2
and CH
4
production are about
Fig. 8
Fe(III) reduction, CO
2
production, and CH
4
production in oxidized (
a
,
b
) and reduced (
c
,
d
) Talladega wetland sediment slurries.
Data source
Roden and Wetzel (
1996
)