Environmental Engineering Reference
In-Depth Information
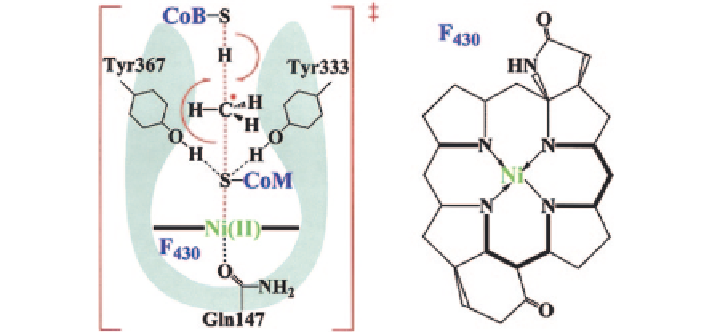
Fig. 5
The mechanism for methane formation in methyl-coenzyme
M
reductase (MCR) in
methanogenesis.
Data source
Pelmenschikov et al. (
2002
)
free methyl radical at the transition state (Fig.
5
). A methyl radical is then released
from methyl-CoM, induced by the attack of Ni(I) on the methyl-CoM thioether
sulfur, which oxidizes the metal center from Ni(I) to Ni(II). The latter forms a
strong bond of 38.6 kcal/mol with the sulfur of CoM (Eq.
2.44
):
COB-S-H + CH
3
-S-COM + NI(I)F
430
→
COB-S-H + CH
3
+ COM-S-NI(II)F
430
(2.44)
The resulting methyl radical is rapidly quenched by hydrogen-atom transfer
from the CoB thiol group, yielding the CH
4
and the CoB radical. The pathway has
activation energy of approximately 20 kcal/mol, leading to stereoinversion at the
reactive carbon (Eq.
2.45
):
(2.45)
COB-S-H + CH
3
+ COM-S-NI(II)F
430
→
CH
4
+
COB-S
•
+ COM-S-NI(II)F
430
In the final step, formation of heterodisulfide CoB-S-S-CoM is proposed in
which nickel is reduced back to Ni(I) (Eq.
2.46
).
CoB-S
•
+ CoM-S-NI(II)F
430
→
CoB-S-S-CoM + NI(I)F
430
(2.46)
It can be noted that methyl-coenzyme M is 2-mercaptoethanesulfonic acid that
is unique to the methanogens, and coenzyme B is 7-mercaptoheptanoylthreonine
phosphate that includes an aliphatic linker of six methylene units between the
phosphothreonine head group and the thiol group.
A recent study shows that MCR is the key enzyme in methane forma-
tion by methanogenic
Archaea
when it is incubated with the natural substrates