Environmental Engineering Reference
In-Depth Information
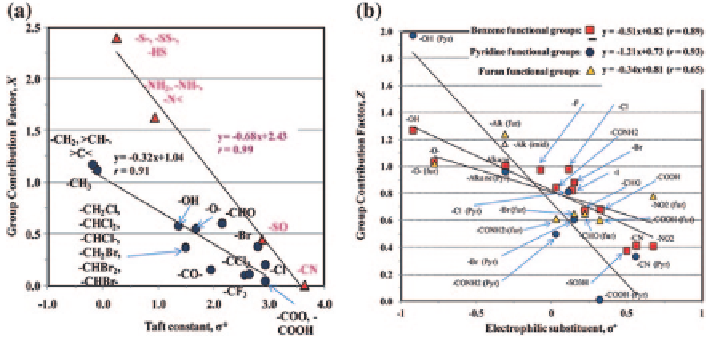
Fig. 2
Comparison of the group contribution factors for H-atom abstraction with the Taft con-
stant,
σ
*
(a; Karelson 2000) and those for HO
•
addition to aromatic compounds with electro-
philic substituent parameter,
σ
+
(Fig. b; Karelson 2000. Group contribution factors include
●
alkyl, oxygenated, and halogenated functional groups and
S-, N-, or P-atom-containing func-
tional groups (Fig. a). Group contribution factors for benzene (
▲
)
compounds (Fig. b). The
σ
*
of [-CHCl
2
], [-CO], [-COO, COOH], [-S-, -SS-, HS-], [-NH
2
,
-NH-, -N <] is an average of [CH
2
Cl, CH
2
Br, CHCl
2
, CHBr
2
], [COCH
3
, COC
2
H
5
, COC(CH
3
)
3
,
COC
6
H
5
, COF, COCl], [COOH, COOC
2
H
5
], [SCH
3
, SC
2
H
5
, SCH(CH
3
)
2
], and [NHCH
3
,
NH(CH
2
)
3
CH
3
, N(C
2
H
5
)
2
], respectively. The
σ
*
of [-SO] and [-N-CO-] refer to [S(O)CH
3
] and
[NHCOC
6
H
5
], respectively.
Data source
Minakata et al. (
2009
)
▪
), pyridine (
●
), and furan (
▲
H-atom abstraction from the O-H bond in methanol, ethanol, and other alcohol
compounds, respectively, which is comparable with the experimental observations
(Asmus et al.
1973
). The
k
-COOH
is 7.0
×
10
5
M
−
1
s
−
1
, which is consistent with
experimental data for oxalic acid (Getoff et al.
1971
).
It is demonstrated that the group contribution factors for the H-atom abstraction
linearly correlate with the Taft constant,
σ
* (Karelson
2000
) (Fig.
2
). The alkyl
functional groups may often weaken the C-H bond with release of the steric com-
pression. The alkyl functional group moves apart to form a planar radical, thereby
increasing the HO
•
reactivity in the H-atom abstraction reactions. Therefore,
X
-CH3
and
X
-CH2-
≈
X
>CH-
≈
X
>C<
values are greater than 1.0, which correspond
to negative values of the Taft constant (Fig.
2
). In contrast, low values of the group
contribution factors for any functional groups indicate their electron-withdrawing
ability (
σ
* > 0).
Rate constant for HO
•
addition to alkenes
(Minakata et al.
2009
): The
•
detailed mechanisms of HO
addition to alkenes in the aqueous phase are not well
documented in earlier studies (Getoff
1991
; Billamboz et al.
2010
). It is gener-
ally considered that
π
-electrons in alkene compounds (>C
=
C<) absorb radia-
tion to form an excited state, which then releases electron (e
−
) to form H
2
O
2
Hydrogen Peroxide and Organic Peroxides in Natural Waters
”
, Eqs. 2.13-2.18).
The HO
•
then reacts with C
+
to form the reaction intermediates. The excitation of