Environmental Engineering Reference
In-Depth Information
etc. The major sources of free radicals such as the superoxide ion (O
2
-
) and
the hydroperoxyl radical (HO
2
•
) are modest leakages from the electron trans-
port chains of mitochondria, chloroplasts and the endoplasmic reticulum
(Miller et al.
1990
; Paradies et al.
2000
; Blokhina et al.
2003
). The HO
•
,
•
•
alkoxyl radical (RO
), and H
2
O
2
produced from the
autooxidation of biomolecules such as ascorbate, catecholamines or thiols, can
damage the macromolecules such as DNA, proteins and lipids in biological
systems (Miller et al.
1990
; Buettner and Jurkiewicz
1996
; Cadet et al.
1999
;
Blokhina et al.
2003
; Berlett and Stadtman
1997
; Morse et al.
1977
; Radtke
et al.
1992
; Abele-Oeschger et al.
1994
). These events have implications for
numerous human health problems. Autoxidation reactions would mostly be
catalyzed by transition metal ions (Fe
2
+
, Cu
+
), and by semiquinones which
can act as electron (e
-
) donors (Buettner and Jurkiewicz
1996
; Blokhina
et al.
2003
). Four-electron reduction of oxygen in the respiratory electron
transport chain (ETC.) is generally associated with a partial one- to three-elec-
tron reduction, yielding reactive oxygen species such as O
2
), peroxyl radical (ROO
•
-
, HO
•
, H
2
O
2
, sin-
•
-
and HO
2
•
glet oxygen (
1
O
2
) and O
3
(Blokhina et al.
2003
). Both O
2
undergo
spontaneous dismutation to produce H
2
O
2
. In the presence of reduced transi-
tion metals such as Fe
2
+
or its complexes that are quite common in biological
systems, the HO
•
radical can be produced by the Fenton reaction. A mecha-
nistic scheme for the generation of HO
•
in biological systems can be depicted
as follows (Eqs.
6.1
-
6.3
) (Buettner et al.
1978
; Buettner
1987
; Buettner and
Jurkiewicz
1996
; Blokhina et al.
2003
):
FE
3
+
-CHELATE
+
O
2
•−
/
ASCH
•
→
FE
2
+
-CHELATE
+
O
2
/
ASC
•−
(6.1)
FE
2
+
-CHELATE
+
H
2
O
2
→
FE
3
+
-CHELATE
+
OH
−
+
HO
•
(6.2)
BIOMOLECULES
+
HO
•
+
O
2
→
FE
3
+
-CHELATE
+
OH
−
+
HO
•
(6.3)
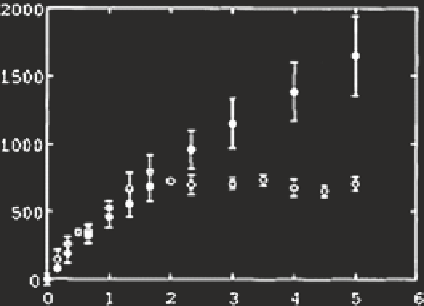
Fig. 9
H
2
O
2
concentration
during photoirradiation of
Suwannee River Fulvic Acid
(10 mg L
−
1
) solutions in
the absence (
•
, average of
five experiments) and the
presence (
, average of three
experiments) of Fe (10
μ
M)
at pH 6.0.
Data source
Southworth and Voelker
(
2003
)
○
Irradiation times (hours)