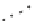Environmental Engineering Reference
In-Depth Information
Fig. 6
Quantum yields of
HO
Wavelength (308 nm)
Wavelength (351 nm)
1.1
•
of H
2
O
2
photolysis with
variation in temperature at
specific wavelengths (308
and 351 nm, respectively) at
pH 7.
Data source
Zellner et
al. (
1990
)
1
0.9
0.8
0.7
275
280
285
290
295
300
Temperature (ºK)
•
Table 6
Quantum yield of HO
production (
Φ
HO
) from H
2
O
2
photolysis at pH 7 as a function of
temperature (T)
T (ºK)
Φ
HO
at pH 7
308 nm
351 nm
278
0.82
±
0.03
0.83
±
0.05
283
0.86
±
0.09
0.88
±
0.06
288
0.91
±
0.04
0.90
±
0.06
293
0.93
±
0.07
0.93
±
0.08
298
0.98
±
0.03
0.96
±
0.04
Data source
Zellner et al. (
1990
)
where the activation energy is 5.5
±
1.6 kJ mol
-1
.
These values are essentially constant for H
2
O
2
photolysis at 351 nm (Eqs.
4.16
,
4.17
):
Φ
HO
(
298 K
) =
0. 96
±
0. 04
(4.16)
1
298
−
1
T
(4.17)
Φ
HO
(
T
) = Φ
HO
(
298 K
)
EXP
(
580
±
160
)
A recent study has shown that the quantum yield
Φ
HO
=
1.11
±
0.07 in the
excitation range of 205-280 nm for the photolysis of H
2
O
2
. This is in agreement
with earlier studies (Goldstein et al.
2007
). Therefore, the available data suggest
that the photolysis of aqueous H
2
O
2
at wavelengths
≥
300 nm generates HO
•
with
a quantum yield
Φ
HO
~1 at room temperature (25 °C).
Φ
OH
decreases to approxi-
mately 0.82 at 5 °C (278 K). It can be noted that the effective quantum yield of
H
2
O
2
dissociation is approximately 0.5, but the photolysis of H
2
O
2
yields two