Environmental Engineering Reference
In-Depth Information
(a)
(b)
1800
2000
1000
200
H
2
O
2
HO
•
1500
800
1500
150
1200
600
900
1000
100
400
600
500
50
200
300
0
0
0
0
0
175
350
525
700
0
175
350 25
700
(c)
(d)
500
100
500
100
400
400
75
75
300
300
50
50
200
200
25
25
100
100
0
0
0
0
0
175
350
525
700
0
175
350 25
700
Irradiation time (min)
•
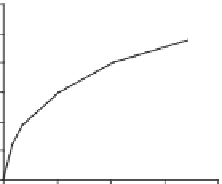
Fig. 2
In-situ generation of H
2
O
2
and HO
for river waters and standard organic substances
during the 10 h of irradiation period in photoexperiments conducted using a solar simulator.
Upstream DOM having mostly fulvic acid (
a
); polluted river waters, mostly affected by mixture
of sewage effluents and upstream DOM (
b
); standard Suwannee River Fulvic Acid (
c
); and stand-
ard diaminostilbene (DAS1) (
d
).
Data source
Mostofa KMG and Sakugawa H (unpublished data)
et al.
2004
; Mostofa KMG and Sakugawa H, unpublished data; Nakatani et al.
2004
). Therefore, the generation of H
2
O
2
by DOM could account for most of the
production of HO
•
by unpolluted water samples, with a relatively elevated content
of fulvic acid in DOM (Fig.
3
) and a relatively low concentration of other HO
•
sources, such as nitrate, nitrite and Fe.
3.2 Direct Photolysis of Nitrate and Nitrite
•
The direct photolysis of nitrite and nitrate induces HO
photoproduction (Zafiriou
and True
1979a
,
b
; Takeda et al.
2004
; Zepp et al.
1987
; Mack and Bolton
1999
).
There is evidence that irradiation in the 200-400 nm wavelength region can con-
vert NO
2
•
•
-
(Eqs.
3.3
,
3.4
) (Zepp et al.
1987
; Mack and Bolton
−
into NO
and O
1999
):
(3.3)
NO
2
+
h
υ →[
NO
2
]
∗
[
NO
2
]
∗
→
NO
•
+
O
•−
(3.4)