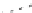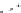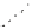Environmental Engineering Reference
In-Depth Information
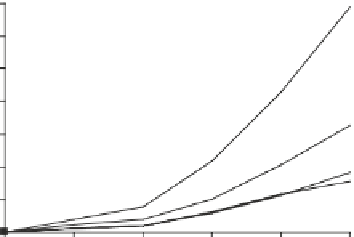
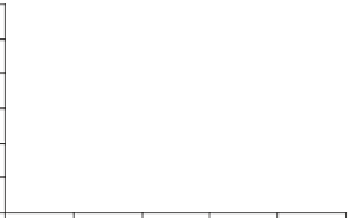
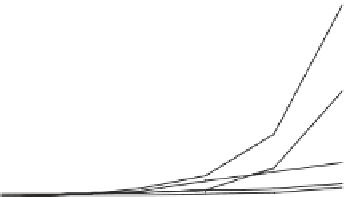
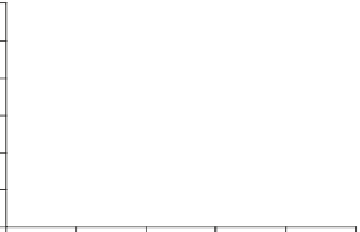
Fig. 1
Photoinduced
generation of HO
•
from river
waters (
a
), various standard
organic substances (
b
)
and various (inorganic and
organic) chemical species
(
c
) in photoexperiments
conducted using a solar
simulator. Aqueous solutions
(1 mg L
−
1
) of standard all
organic substances are used
for production of HO radicals
in (
b
) and all chemical
species in (
c)
are adjusted to
100
μ
M. All data depicted in
these figures are calibrated
for natural sunlight on 6 July
2004 at Hiroshima University
Campus at noon under clear
sky conditions.
Data source
Mostofa KMG and Sakugawa
H (unpublished data)
(a)
upstream water (KR1)
upstream water (KR2)
downstream water (KR3)
downstream water (KR4)
downstream water (KR5)
downstream water (KR6)
Milli-Q water
14000
12000
10000
8000
6000
4000
2000
0
0
30
60
180
360
600
(b)
SRFA
SRHA
Tryptophan
Phenylalanine
DSBP
DAS1
Milli-Q water
1200
1000
800
600
400
200
0
0
30
60
180
360
600
(c)
Hydrogen peroxide
Peracetic acid
Nitrite
Nitrate
Sulphate
Chloride
Milli-Q water
30000
25000
20000
15000
10000
5000
0
0
30
60
180
360
600
Irradiation time (min)
H
2
O
2
, H
2
) (Henglein
1987
); (xiii) autooxidation of aqueous extracts of cigarette tar
(ACT), giving HO
•
in air-saturated, buffered aqueous solutions. It is thought that the
process is caused by the autooxidation of hydroquinone- and catechol-related species
in ACT (Zang et al.
1995
); (xiv) photoinduced HO
•
production from aqueous suspen-
sions of algae(Li et al.
2008
); and (xv) photoinduced HO
•
production can occur from
DOM, the reactive triplet states of which could be involved in oxidation of water and/
or OH
-
and in the production of lower energy hydroxylating species that simulate
DOM reactivity (Alegria et al.
1997
; Pochon et al.
2002
; Gan et al.
2008
; Maurino
et al.
2008
; Maddigapu et al.
2010
; Page et al.
2011
; Maddigapu et al.
2011
; Brigante
et al.
2010
; Sur et al.
2011
).