Environmental Engineering Reference
In-Depth Information
Upstream waters
Downstream waters
SI
SI
80
3.0
70
2.5
60
2.0
50
40
1.5
30
1.0
20
0.5
10
0
0.0
August 21, 2003
September 26, 2003
Sampling period (Japan standard time, JST)
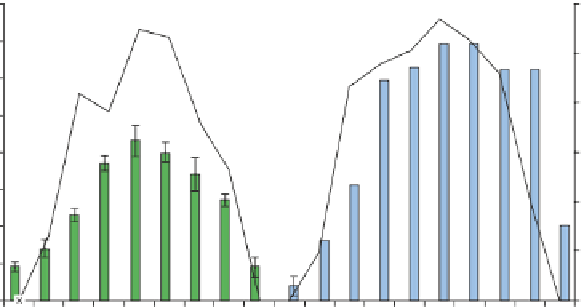
Fig. 6
Diurnal variations of H
2
O
2
concentrations in the upstream waters (site KR2) on
21August 2003 and in the downstream waters (site KR6) on 26 September 2003, in the Kurose
River.
Data source
Mostofa and Sakugawa (
2009
)
of Aqaba (Herut et al.
1998
), 32 nM in Lagrangian-Atlantic Ocean, 20 nM in
Underway-Atlantic Ocean (Yuan and Shiller
2001
), 59 nM in Bermuda, Atlantic
Time Series Station (Avery et al.
2005
), 491 in a shallow freshwater stream
(Richard et al.
2007
), and 365 nM in marine bathing waters at Huntington State
Beach (Clark et al.
2010
).
The magnitude of the diurnal cycle of H
2
O
2
production shows seasonal and
spatial variations in natural waters, depending on several factors. First, the solar
intensity varies greatly among tropical, sub-tropical, Arctic and Antarctic regions.
The diurnal cycle of H
2
O
2
is in fact the best paradigm for the dependence of its
production on solar intensity. Second, the contents and nature of DOM compo-
nents are widely different for a variety of waters and cause correspondingly
variable production rates of H
2
O
2
. For example, H
2
O
2
concentration is almost
doubled in waters having high DOC concentration (326-384
μ
M C) than in
waters with low DOC (118-239
μ
M C), even in the presence of similar solar irra-
diance (Mostofa and Sakugawa
2009
). A third factor is the presence of catalase
and peroxidase enzymes associated with microbes or algae. Biological processes
are widely variable for a variety of natural waters and can control the steady-
state concentration by rapidly decomposing H
2
O
2
(Fujiwara et al.
1993
; Petasne
and Zika
1987
; Moffett and Zafiriou
1990
; Mostofa (Manuscript in preparation).
Fourth, iron (Fe) can reduce the steady-state H
2
O
2
concentration by producing
HO
•
through the photo-Fenton or other photoinduced reactions in natural waters
(Moffett and Zafiriou
1990
; Zepp et al.
1992
; Southworth and Voelker
2003
).