Biology Reference
In-Depth Information
increase in charge intensity and dispersal over evolutionary time reflected a trend
toward increasing protein binding specificity.
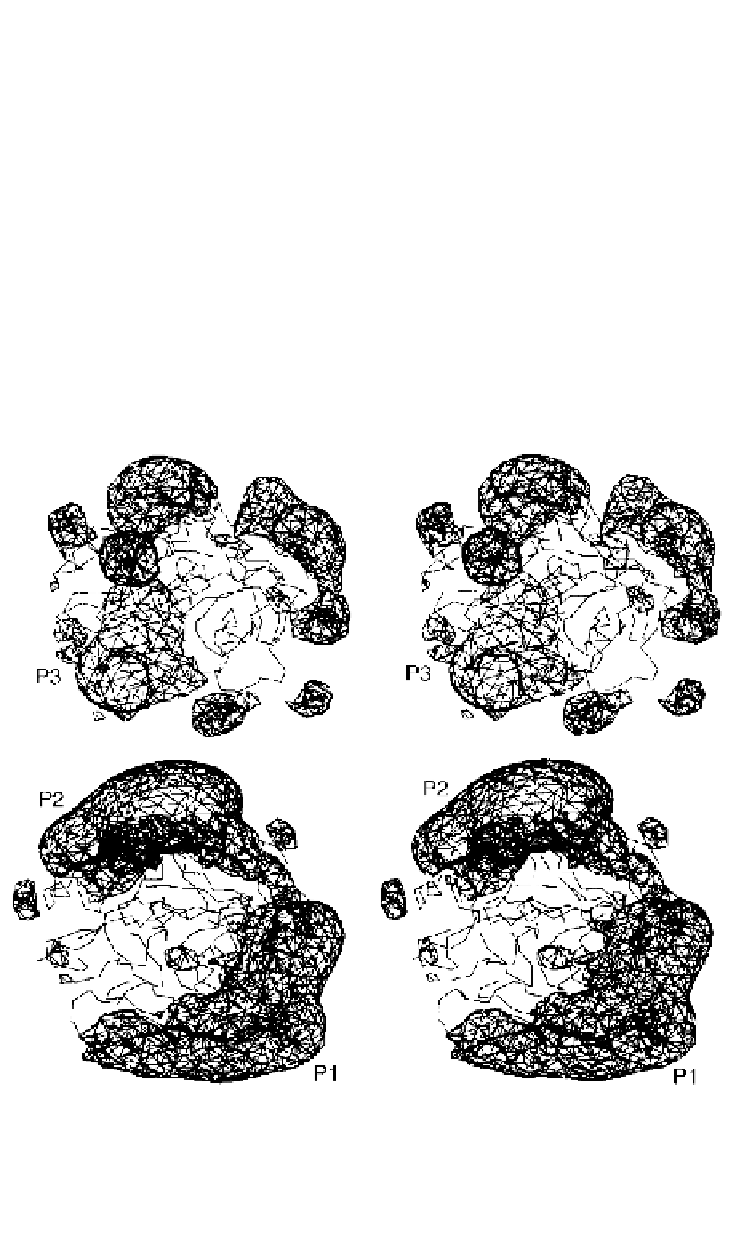
The fibrinogen-binding exosite (P1) was found to have greatly increased in size
during the evolution of thrombin. From the electostatic contour map, only five
Arg (126, 165, 233) and three Lys residues (230, 243, 245) of the putative ancestral
protein appeared to contribute to a small patch of positive charge (
Figure 10.2
).
Moreover, several thrombin residues known to be important in the binding of fib-
rinogen (Tsiang
et al
., 1995) were not present in the ancient protein suggesting
that the ancestral protease bound fibrinogen only weakly (
Figure 10.3
). A number
of residues in thrombin have been shown to be involved in the binding of protein
C (Tsiang
et al
., 1995) and these are also components of the fibrinogen binding site
(Lys36, Trp60D, Lys70, His71, Arg73, Tyr76, Arg77A, Lys81, Lys109, Lys110,
Glu217, Arg221A;
Figure 10.3
) . However, only a fraction of these residues were
present in the ancestral protein (Trp60D, His71, Arg77A, Lys110, Glu217,
Arg221A). This was not surprising since, at the dawn of vertebrate evolution, pro-
tein C had yet to evolve from the vitamin K-dependent factor ancestral protein
Figure 10.2.
Stereo views of the electrostatic profiles of the putative vitamin K-dependent
factor ancestral protein (above) and extant human thrombin (below). The view is towards
the active site canyon. The positions of the fibrinogen-binding site (P1) and heparin-
binding site (P2) are indicated. A large positively charged patch (P3) is also present on
the vitamin-K dependent factor ancestral protein (after Krawczak
et al.
, 1996).