Chemistry Reference
In-Depth Information
PLGA: Poly (D, L-lactide-co-glycolic acide) 50:50
MION: Monocrystalline iron oxide nanopartticles
PVA: Poly (vinyl alcohol)
MION solution
Stir with
isopropyl alcohol
Homogenization
with PVA
Sonication
PLGA in ethyl acetate
Water/oil emulsion
(Water/oil) water/emulsion
Lyophilization
Centrifugation
Microspheres
MION-entrapped PMB
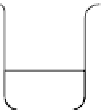
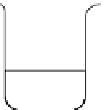
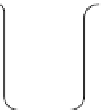
fIgure 14.7
Flow diagram representing the method for synthesising iron oxide nanoparticles entrapped polymeric microbubbles to
increase microbubble MR detectability. (Reproduced from Ref. [104], with permission from Wiley).
14.4.3
Increasing Microbubble Mr detectability
Microbubble susceptibility effects are relatively weak when compared with other intravascular MR susceptibility contrast
agents. Given the possible microbubble toxicity at high dosage and the filtering of microbubbles larger than 10 μm by the
lung capillary bed [97, 102], microbubble volume fraction and radius cannot be freely chosen. Without increasing static field
strength, enhancement of overall magnetic susceptibility difference between the microbubble and the blood plasma is a
preferred way to increase microbubble-induced signal perturbation.
Effective microbubble magnetic susceptibility can be manipulated by changing the shell thickness and the magnetic
susceptibility of the shell or filling gas [102]. because the type of filling gas is chosen mainly to improve the microbubble
stability
in vivo
, modifying the magnetic susceptibility of the shell is a preferred means to increase the microbubble
magnetic susceptibility. Theoretical studies have indicated that, by embedding or coating magnetic nanoparticles, the
magnetic susceptibility of the shell can be increased [94, 102], thus enhancing the microbubble susceptibility effect and
alleviating the dosage requirement for MRI applications (Figure 14.7). It was experimentally demonstrated that gas-filled
polymeric microbubble susceptibility effects can be substantially enhanced by incorporating iron oxide nanoparticles into
microbubble shells through phantom and rat studies [104, 105]. Such microbubbles loaded with oxide nanoparticles were
also reported to possess increased acoustic backscattering and longer MR contrast enhancement [106, 107], suggesting
its potential application as a dual contrast agent in US and MRI.
Microbubbles filled with hyperpolarised
3
He gas provide an alternative to increase MR signal, allowing acquisition of
angiography and perfusion images with higher signal-to-noise ratio [108, 109]. However, it requires hardware tuning for
signal reception at
3
He resonance frequency and may possibly hinder its clinical applications.
14.4.4
future applications
As discussed previously, microbubbles can be locally cavitated and destroyed by focused US [5]. Therefore, their MR sig-
nals can be temporally and spatially manipulated because microbubble disappearances will diminish the susceptibility effect,
as confirmed in a phantom study [110]. Without causing microbubble sonoporation and cavitation, MRI visualisation may
provide the most effective imaging guidance for microbubble-mediated genes or drug delivery. Incorporating gadolinium/
manganese chelates into microbubble shells may also improve their susceptibility effect. Upon cavitation, the entrapped
paramagnetic chelates could be released and in contact with surrounding water molecules, thus shortening the
T
1
relaxation
time and producing increased signal in
T
1
-weighted imaging. Such dark-to-bright change could be a potentially attractive
way to monitor microbubble-based drug delivery and therapeutic applications.
Moreover, due to the capability of being locally cavitated, microbubbles possess potential blood-labelling applications
with perfusion analysis [111]. Due to the hyperpermeable nature of tumour neo-vessels as compared with normal vessels
[112], microbubbles may be able to pass through tumour vessels and therefore accumulate in the tumour tissues, producing