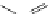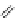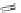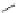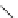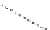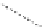Chemistry Reference
In-Depth Information
O
N
O
P
N
N
Tb
O
C
4
H
9
O
P
N
O
OC
4
H
9
O
P
O
OC
4
H
9
FigUre 12.15
Bornhop's macrocyclic terbium complex.
12.3.2
examples of
f
f-block Lumophores as imaging Labels
The use of luminescent lanthanide complexes as optical labels in various fluoroimmunoassays has been established for over
30 years and was developed in parallel with their use as labels for cytochemistry and histochemical studies. In a biological
imaging context, specific design criteria must be addressed to ensure that highly emissive, long-lived Ln
III
probes persist in
aqueous environments: The last 10 to 15 years of development has seen this challenge met, and the application of such
species to confocal fluorescence microscopy and time-resolved luminescence microscopy is now rapidly developing [51].
One of the earliest examples of optical imaging using an Ln
III
complex in a biological context was reported in 1999 by
Bornhop. The development of a polyazamacrocyclic Tb
III
complex (Figure 12.15) that incorporates a rigidifying ring-pyridine
donor and phosphonic acid ester arms demonstrated promise as an abnormal tissue marker. Both
in vivo
and
in vitro
imaging
showed that the complex, which has low cytotoxicity, possessed useful optical properties and demonstrated fluorescence
imaging capability at picomolar concentrations in tissues. Adenocarcinoma cells showed good affinity for the complex,
enabled by the lipophilic nature of the ligand periphery and the overall neutral charge of the complex, as demonstrated
through
in vitro
fluorescence detection and histological assessments [52].
More recently gunnlaugsson has extended the utility of emissive Ln
III
-based complexes to the imaging assessment of
damaged bone structure (specified as microcracks). An emissive, dO3A-type Eu
III
complex (Figure 12.16), functionalised
with an amido-naphthalene antenna and peripheral iminodiacetate groups, can effectively target exposed Ca
II
sites of the
hydroxyapatite lattice of the bone. Steady state luminescence spectroscopy was used to identify the binding of the Eu
III
com-
plex to a scratched (i.e., damaged) bone surface, through observed changes in the ratiometric emission of the Eu
III
. The
application of confocal fluorescence microscopy (Figure 12.16) demonstrated the potential for revealing far greater fine detail
of the bone surface morphology through the observation of Eu-based visible emission and improved signal contrast [53].
An assessment of binding events at cell surfaces can be probed with luminescent labels. Both Tb
III
and Eu
III
complexes of
chromophore-appended dO3A-type ligands have been designed to target the lipophilic plasma membrane of cells by incor-
porating long alkyl chains into the ligand framework. The amphiphilic complexes (Figure 12.17) appear to bind to the cell
surface, presumably via the alkyl chains, and can be imaged using confocal fluorescence microscopy. The use of both Eu
III
and Tb
III
complexes to dual-label the cell provides further possibilities for interrogating the cell surface by studying the
distance-dependent intermolecular energy transfer processes that can occur between the Ln
III
ions. In this case, the energetic
pathway favours Tb
III
-sensitised-Eu
III
emission, through observation of the relative quenching of the donor component (Tb
III
)
versu
s
the acceptor (Eu
III
) [54].
12.3.3
examples of time-resolved imaging with
f
f-Metal Labels
As discussed earlier, time-resolved luminescence microscopy and/or FLIM allow for very effective removal of autofluo-
rescence and improve the imaging sensitivity of the microscopy study. For maximal resolution, the lifetime of the lumophore
should preferably reside in the microsecond-to-millisecond domain; emissive Ln
III
complexes represent ideal candidates
for this purpose. The application of Ln
III
complexes to time-resolved luminescence microscopy has been demonstrated
through labelling of small silica particles with cationic, chromophore-functionalised dO3A-type complexes of Eu
III
(Figure 12.18). These particles were compared to those labelled with rhodamin 6g (i.e., a short-lived fluorophore), which
absorbs and emits at coincident wavelengths to that of the Eu
III
complex. The microscopy studies demonstrated that
employing a microsecond time-delay allowed the Eu-labelled silica particles to be easily distinguished from those labelled
with rhodamin 6g [55].
A demonstration of these optical advantages has been shown in a biological context, where imaging experiments on a
fluorescein-labelled antibody and a Tb
III
-labelled BSA conjugate were conducted, comparing the prompt fluorescence (in
essence, total emission) with time-resolved (0.5 ms time delay) luminescence imaging. The latter revealed a 1000-fold
increase in the Tb
III
/fluorescein emission ratio, demonstrating the utility of biospecific probes based on emissive Ln
III
chelates [56].