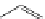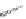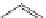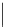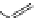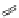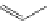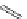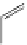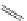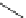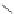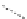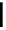Chemistry Reference
In-Depth Information
membrane permeancy on the imaging agents, but these are best treated by class of metal as the design criteria of the
d
- and
f
- block cases are markedly different.
12.1.1
d
- and
f
-block imaging Agents
The
d
-block (the transition metals) offers a vast range of molecular complexes that have suitable properties for application as
agents in fluorescence cell imaging, with luminescent examples of complexes of almost all metals having been reported.
However, as yet there are only a small number of types of complex that have been so used, and even fewer that have gained
popularity. The most widely studied families of
d
-block imaging agents are those based around second- and third-row transition
metals complexes bearing conjugated aromatic ligands in which the metal is in the
d
6
configuration [3]; however, there are also
a number of examples of other types of complex, notably
d
8
and
d
10
complexes of the platinum group metals [4]. The trivalent
lanthanide ions (Ln
III
) of the
f
f-block also provide many opportunities [5] for consideration as optical cellular imaging agents.
Unlike the
d
6
transition metal ion complexes, which benefit from an inherent and advantageous kinetic inertness, a significant
challenge with Ln
III
based systems is addressing the issue of kinetic stability in aqueous, and more pertinently, physiologically
relevant environments: typically this requires encapsulation of the Ln
III
within a multidentate ligand array. Unlike the more
rigid coordination geometries imposed by the
d
6
electronic arrangement, the Ln
III
ions typically experience much weaker
ligand field preferences and higher coordination numbers. In this context, the design of effective ligand systems for Ln
III
reflects the predominantly electrostatic nature of bonding between Lewis acidic Ln
III
ions and hard ligand donor moieties.
12.2
d
6
MetAL CoMPLexes in FLUoresCent CeLL iMAging
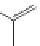
Several common features are shared by the three main families of
d
6
lumophores, that is, iridium cyclometallates, rhenium
fac
tricarbonyl polypyridyls, and ruthenium trisbipyridyls (Figure 12.1a-c, respectively) that make them attractive for cell imaging:
(a)
Photophysics:
All show excitation in the visible region of the spectrum, large (100 s of nm) Stokes' shifts and long
(100-1000 ns) luminescence lifetimes, resulting from emission from triplet excited states with variable degrees of
MLCT and IL character.
(b)
Photostability:
All show reduced photobleaching compared to common organic fluorophores.
(c)
kinetic stability toward ligand exchange:
Heavy metal toxicity is largely associated with interactions of biomole-
cules with the metal centres, and so these coordinatively saturated complexes with very slow rates of ligand exchange
typical of the
d
6
low-spin configuration prevent these interactions and thus reduce toxicity.
12.2.1
Photophysics of
d
6
Polypyridyl Lumophores
The photophysical properties of
d
6
lumophores are complex with variation between specific examples, but there is a general
theme of triplet metal-to-ligand-charge-transfer (
3
MLCT) that is important in the majority of examples. Briefly, this involves
(Figure 12.2a) excitation by a photo-induced electron transfer from metal-based orbitals to a conjugated π-system usually
located on an aromatic heterocyclic ligand (often pyridyl). This generates a singlet excited state (
1
MLCT) that is rapidly con-
verted
via
intersystem crossing (Figure 12.2b) to the triplet excited state, from which emission (Figure 12.2c) occurs. It is impor-
tant that other metal-based orbitals are not available for electron transfer from the excited state, because this can lead to energy
loss through pathways that do not involve emission of light. For this reason the complexes usually include
high-field
ligands that
will ensure that the vacant
d
-orbitals are of too high an energy to become involved in any of the deactivation processes. Because
the excited state therefore involves charge transfer from metal to ligand, the metal should be easily oxidised, and the ligand
+
2+
+
N
N
N
OC
N
N
N
Ir
Ru
Re
N
N
N
OC
N
CO
2
2
1A
1B
1C
FigUre 12.1
Common structural types in
d
6
imaging agents.